Published on
Urgent message: Rhabdomyolysis has a wide range of presentations, from asymptomatic to life-threatening. The most dramatic presentation can result in acute renal failure, electrolyte imbalances, and/or disseminated intravascular coagulation (DIC).
Jordan Miller, DO, Ari Leib, MD, and Andre Bonnet, DO
EPIDEMIOLOGY
Approximately 26,000 cases of rhabdomyolysis are reported in America yearly, with 10% to 50% progressing to acute renal failure.1,2 Mortality rates range from 7% to 80% and are higher in patients who develop multiorgan failure.3 While the most common cause is direct muscle trauma, a wide range of processes precipitate, including autoimmune/inflammatory myopathies.1,2
Causes of rhabdomyolysis include:
- Heat-related events, such as heatstroke and marathon running
- Trauma, such as immobilization and crush injuries
- Malignant hyperthermia, neuroleptic malignant syndrome (NMS), and other toxicological events
- Endocrine problems, such as hypothyroidism, thyrotoxicosis, diabetic ketoacidosis (DKA)
- Environmental events such as lightning strikes and third-degree burns
- Inflammatory processes such as polymyositis and dermatomyositis.4,5
The prevalence of polymyositis and dermatomyositis is 5 to 22 per 100,000 patients, and the incidence is approximately 1.2 to 19 million persons at risk per year.6 Dermatomyositis is an acquired muscle disease which results in chronic muscle inflammation and weakness. A classic skin rash may precede the muscle weakness. It is most commonly seen in adults between the ages 40 and 60.6
Most patients experience muscle aches and weakness of the more proximal muscles, which results in difficulty performing certain activities such as raising their arms over their head, climbing stairs, and swallowing. A reddish-purple heliotrope rash may develop over the eyelids and across the cheeks and bridge of the nose. Gottron papules can develop over the knuckles, elbows, knees, and other extensor regions; this appears as a scaling and red rash.7
Polymyositis is another acquired muscle disease that is also characterized by chronic muscle inflammation and degenerative changes, which leads to symmetric weakness and proximal muscle atrophy. As mentioned above, the common areas affected are the proximal muscle groups. Polymyositis usually begins around the age of 20, has a gradual progression, and is more common in women than men.8 Unlike dermatomyositis, there are no skin changes. Risk of developing polymyositis is higher in patients with other autoimmune disorders such as systemic lupus erythematous (SLE), rheumatoid arthritis, scleroderma, and Sjogren’s syndrome. Complications of polymyositis include difficulty swallowing and aspiration. Patients may experience dysfunctional breathing if chest muscles are affected, which may lead to shortness of breath and eventual respiratory failure.9 A history suggesting one of the polymyopathies must heighten the provider’s concern for the risk of rhabdomyolysis.
CASE REPORT
History
A 52-year-old-male presented with complaints of generalized pain, which he said had occurred in the past, once culminating in acute renal failure with an associated serum creatinine kinase of >20,000 units/L. His pain was most pronounced in his calves, extremities, and lower back. He denied any recent trauma but had been doing work around the house and riding his bike. He said that his urine had become much darker. He complained of weakness but denied any chest pain, shortness of breath, nausea, vomiting, diarrhea, or skin changes. He admitted to drinking about six liters of water a day to help with his pain because he had the episode of acute renal failure previously.
Exam
Physical exam revealed a disheveled male who appeared older than his stated age. His vital signs were notable for being afebrile, with a blood pressure of 110/60, a heart rate of 96, respiratory rate 16, and an oxygen saturation of 100% on room air. His cardiovascular, pulmonary, and abdominal examination was unremarkable. He had decreased strength to bilateral thighs and decreased ability to dorsiflex the left foot. He had excruciating tenderness to the calves and lower back with palpation. Pulses were 2+ equal in all extremities. No rashes were seen throughout on his integumentary examination.
Differential Diagnosis
The differential diagnosis for myalgia and dark urine includes:
- Renal colic/nephrolithiasis – Rhabdomyolysis may be confused with renal colic and both may have a urine dipstick positive for blood. However, urolithiasis is not associated with marked elevations of creatinine kinase and myoglobinuria is not present in renal colic
- Guillain Barré syndrome – Often presents with ascending muscle weakness. Monitoring the negative inspiratory fraction (NIF) and vital capacity (VC) is important in these patients. Diagnosis is achieved with lumbar puncture and analysis of cerebrospinal fluid for cytoalbumin dissociation and electrophysiologic evidence of demyelination
- Nonaccidental trauma or neglect
- Cold or heat exposure
- Dehydration
Testing/Outcome
Laboratory evaluation in the emergency room revealed a potassium level of 4.3, elevated serum creatinine kinase (CK) of >20,000 units/L and elevated ALT at 330 units/L and AST at 680 units/L. Renal function panel revealed a creatinine of 0.50 mg/dL and a BUN of 15 mg/dL. A right upper quadrant ultrasound revealed no abnormalities of the liver or gallbladder and the patient denied a history of alcoholism. Urinalysis was negative for infection but showed large blood without red blood cells. He was given two boluses of crystalloid fluid and started on maintenance intravenous fluids.
Further questioning revealed that he had had multiple episodes of rhabdomyolysis, including an episode of acute renal failure. He also reports “self-treating” episodes of rhabdomyolysis at home by increasing oral fluid intake.
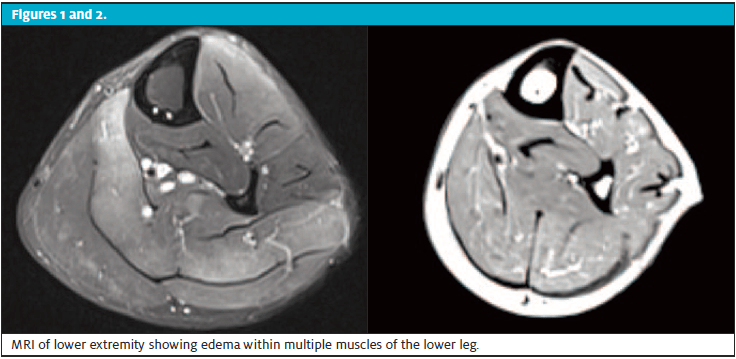
Additional workup revealed an aldolase of 317.1 units/L. MRI of the lower extremity showed edema within multiple muscles of the lower leg, especially within the soleus muscle, but also seen within the anterior and lateral compartments and within the gastrocnemius muscles.
After the MRI, the patient had a muscle biopsy which was reported as skeletal muscle with areas of myofibril degeneration with loss of cross striations and increased interstitial inflammatory cells composed predominately of mononuclear type cells. Based on these tests, he was diagnosed with polymyositis, which offered explanation for his recurrent rhabdomyolysis. Further laboratory analysis demonstrated a positive SSO-A/Ro 52 antibody which returned elevated at 124 AU/mL. The patient was monitored in the hospital until his CK levels improved. He was discharged with advice for rheumatology specialty follow-up.
DISCUSSION
Rhabdomyolysis is a syndrome where the body breaks down skeletal muscle fibers, which subsequently causes actin and myosin fibers to become necrotic. Eventually leakage of muscle contents into the circulation occurs. Common causes include crush injury, excessive physical exertion, alcohol abuse, and certain medications associated with syndromes of toxicity such as neuroleptic malignant syndrome (NMS). Genetic disorders have also been implicated as precipitating factors of rhabdomyolysis (such as in muscular dystrophy and glycogen storage diseases). Inflammatory myopathies such as polymyositis and dermatomyositis, as seen in our patient, can be the precipitating factors behind recurrent episodes of rhabdomyolysis. Features of rhabdomyolysis are often nonspecific.4
Classically, rhabdomyolysis presents as a triad of symptoms that include:
- myalgia
- weakness
- myoglobinuria
However, only 10% of patients are seen to have the classic triad and about half of patients do not complain of muscle pain or weakness at all.4 Instead, patients often present with discolored urine (tea or red in color) as their chief complaint.
Complications of rhabdomyolysis can be categorized into early and late. In the acute phase of rhabdomyolysis, hyperkalemia from severe muscle breakdown can cause cardiac arrhythmia and sudden cardiac arrest within the first 12 hours.1,10 Approximately 15% of patients go on to experience the late complication of renal failure, which is secondary to the toxic effect of myoglobin on the renal tubules.4 Acute renal failure and diffuse intravascular coagulation are late complications that occur within 12-24 hours.4
Diagnosis
Common physical exam findings include:
- Muscle pain and tenderness with decreased muscle strength
- Soft tissue swelling
- Pressure necrosis
- Calf and lower back pain
- Muscle atrophy in the setting of myopathy disease10
Testing should include a creatinine kinase and electrolyte and renal function panel to assess for acute renal dysfunction and elevated potassium. (CK levels help us identify rhabdomyolysis but do not indicate the prognosis.11 Urinalysis will typically show blood on dipstick without red blood cells on microscopic evaluation. An EKG should be performed secondary to evaluation for cardiac toxicity of elevated potassium.
Inflammatory myopathies are diagnosed by characteristic historical and physical exam findings, such as proximal muscle weakness and evidence of effect on activities of daily living (eg, asking the patient if they have difficulty with brushing their teeth or walking upstairs). Obtaining screening labs such as aldolase, ALT/AST, and lactate dehydrogenase (LDH) can help in evaluating for underlying inflammatory myopathies. Although nonspecific, obtaining an erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) can help evaluate for inflammation.
Antinuclear antibody (ANA) is another screening tool commonly used, and obtaining specific antibodies such as Jo-1 antibodies and SSA/SSB can also aid in diagnosis. Further inpatient testing may include electromyography (EMG), which is able to evaluate for skeletal muscle activity during rest and activity. In inflammatory myopathies, this test will be abnormal. MRI can evaluate for inflammation in the muscle groups. The gold standard for diagnosis is with a muscle biopsy, which will show muscle degradation.7,8
Pathophysiology
Rhabdomyolysis involves direct muscle injury or failure of energy supply within the muscle cell, leading to cellular death and lysis. Normally, within the muscle cell there are ion channels that maintain low intracellular sodium and calcium levels with high intracellular potassium concentrations. During depolarization, there is an influx of calcium ions from the sarcoplasmic reticulum into the sarcoplasm, which causes the muscle cells to contract through actin-myosin cross-linking. These processes depend on ATP which, through injury, can disrupt the normal process and cause an imbalance within the muscle cells and electrolyte concentrations. As ATP is depleted, there is an excessive intracellular influx and calcium and sodium ions. As sodium enters the cell, water and thus the intracellular space is further disrupted and cellular swelling results. High calcium ion concentrations cause sustained myocyte contraction and further ATP depletion. Lysis of the cellular membrane occurs and further damages the ion channel, resulting in muscle injury and inflammation. A myolytic process occurs and necrosis of the muscle cell ensues, with subsequent release of the muscle contents into the extracellular space and bloodstream.
Management
Treatment of rhabdomyolysis includes aggressive fluid administration to prevent renal injury. Most data indicate that acute kidney injury doesn’t increase until the CK level exceeds 5,000 units/L.2
Intravenous hydration should be initiated immediately. One study showed that forced diuresis within 6 hours of admission prevented all episodes of acute renal failure. At first isotonic crystalloid should be given in high volume at a rate of 1.5 L per hour and a high urine output should be maintained (300 mL per hour) until myoglobinuria resolves. Maintenance IV fluids should be continued until CK levels are below 1,000 units/L. If oliguria develops despite aggressive fluid resuscitation, switching fluids to 0.45% normal saline with 1 to 2 amps of sodium bicarbonate is appropriate. Additionally, 10 g/L of mannitol can be added for oliguria. If the patient develops severe acute kidney injury (AKI) and/or oliguria, consultation with a nephrologist for consideration of emergent dialysis is prudent.1,4,10
Mannitol and bicarbonate are often used to supplement fluid resuscitation with normal saline. One study showed a protective effect with mannitol due to diuresis, which minimizes intratubular heme pigment deposition.
Mannitol has been shown to act as a free radical scavenger, which allows for decreased cellular injury. Using an alkalizing agent (ie, sodium bicarbonate) has been shown to minimize renal damage; however, there been no prospective randomized control trials that have shown mannitol and bicarbonate to improve outcomes above aggressive fluid resuscitation. A retrospective study of 24 patients showed that administration of normal saline alone prevented the progression to renal failure and that addition of mannitol and bicarbonate showed no additional benefit.12
Dialysis is another cornerstone of treatment modalities for rhabdomyolysis when renal failure and/or refractory hyperkalemia occurs. Patients can develop oliguric acute tubular necrosis which, in turn, can result in acute renal failure.1
Hemodialysis should be started in conjunction with a nephrologist. Many patients with previously normal renal function and AKI due to rhabdomyolysis will recover intrinsic renal function, and not go on to permanent dialysis dependence.1 Various forms of dialysis have been used for acute renal failure if it develops, but there is no evidence to support the use of a specific dialysis modality. It has been hypothesized that the use of dialysis functions to directly reduce circulating myoglobin.12,14
Management of polymyositis and dermatomyositis includes corticosteroids as the initial therapy, especially in episodes of acute exacerbation of inflammation. Prednisone is dosed at 1 to 2 mg/kg/day in most patients. In patients with severe weakness or extramuscular involvement, IV methylprednisolone 1 mg/day for 3 days can be used.14 Patients should be monitored for steroid-related adverse effects such as steroid induced myopathy, weight gain, hyperglycemia, hypertension, adrenal insufficiency, and osteoporosis. For prevention of acute episodes, patients may be started as outpatients on immunologic agents such as rituximab, which is an anti-CD20 monoclonal antibody. Tocilizumab (anti IL-6 antibody), anakinra (anti-IL 1 antibody), and alemtuzumab (anti CD52 antibody) are all options that may be started under the direct care of a rheumatologist.8,15
Disposition/Considerations for Transfer
- Muscle tetany or cramping not relieved with anti-inflammatories and fluids
- Urine dipstick that shows blood without red blood cells
- Findings consistent with SIRS or sepsis
TEACHING POINTS
- Recurrent episodes of rhabdomyolysis often occur due to an underlying autoimmune disorder, such as an inflammatory myopathy.
- Urinalysis will often reveal a dipstick exam positive for blood, but without red blood cells noted on urine microscopy.
- Creatinine kinase above 5,000 units/L is associated with AKI. Myoglobin release from muscle necrosis contributes to renal failure due to direct toxicity to the renal tubular system. Consider admission.
- Treatment of rhabdomyolysis centers around fluid resuscitation. Bicarbonate, and/or mannitol may also play a role in certain cases. Emergent dialysis may be required in severe cases with oliguria nonresponsive to less invasive care.
References
- Sauret J, Marinides G, Wang, G. Rhabdomyolysis. Am Fam Physician. 2002;65(5):907-913.
- Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009; 361(1):62-72.
- Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
- Torres P, Helmstetter J, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Oschsner J. 2015;15(1):58-69.
- Vanholder R, Sever M, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11(8):1553-1561.
- Cheeti A, Panginikkod S. Dermatomyositis and polymyositis. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019.
- Marvi U, Chung L, Fiorentino DF. Clinical presentation and evaluation of dermatomyositis. Indian J Dermatol. 2012;57(5):375–381.
- Hunter K, Lyon MG. Evaluation and management of polymyositis. Indian J Dermatol. 2012;57(5):371–374.
- Johns Hopkins. Health Conditions and Diseases: Polymyositis. Available at: https://www.hopkinsmedicine.org/health/conditions-and-diseases/polymyositis. Accessed March 2, 2020..
- Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67(9):272-283.
- Baeza-Trinidad R, Brea-Hernando A, Morera-Rodriguez S, et al. Creatinine as predictor value of mortality and acute kidney injury in rhabdomyolysis. 2015;45(11):1173-1178.
- Keltz E, Khan F, Mann G. Rhabdomyolysis: the role of diagnostic and prognostic factors. Muscles Ligaments Tendons J. 2013;3(4):303-312.
- Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058-1065.
- Chatzizisis Y, Misirli G, Hatzitolios A, Giannoglou G. The syndrome of rhabdomyolysis: Complications and treatment. Eur J Intern Med. 2008;19(8):568-574.
- Greenberg SA. Inflammatory myopathies: evaluation and management. Semin Neurol. 2008;28(2):241–9.



