Urgent message: After a brief hiatus during the peak of the COVID-19 pandemic, the rate of new sexually transmitted infections has resumed at epidemic rates in the United States. With many specialty STI clinics having closed, urgent care may be better positioned than ever to help curb their spread.
Glenn Harnett, MD
Citation: Harnett G. The rising importance of urgent care in the fight against the STI epidemic. J Urgent Care Med. 2022;16(3):15-20.
Key words: chlamydia, gonorrhea, STD, STI, syphilis
INTRODUCTION
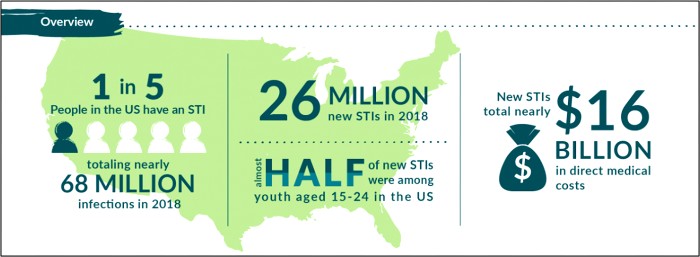
The 2020 CDC STD Surveillance Report showed a decrease in reported sexually transmitted infections during the early months of the COVID-19 pandemic—quickly followed by a resurgence in reported cases of gonorrhea and syphilis that surpassed 2019 levels,1 including more than 2.5 million cases of chlamydia, gonorrhea, and syphilis. Gonorrhea cases rose to 616,392, a 56% increase from 2015, while chlamydia cases rose to 1.8 million, up 19% from 2015.2 Chlamydia cases declined slightly in 2020, but the Centers for Disease Control and Prevention attributed that dip to decreases in screening and underdiagnosis during the pandemic (Figure 1). The American healthcare system incurs nearly $16 billion in direct lifetime medical costs (Figure 2)—just for the new STIs contracted in a single calendar year.3
This surge may be due, in part, to recent advances in the treatment and prevention of human immunodeficiency virus (HIV). The successes against HIV may have caused some to change their attitudes toward condom use and other prevention strategies, with the unfortunate and initially counterintuitive result that reducing the threat of HIV may be accompanied by increasing exposures to other STIs.4 Both chlamydia and gonorrhea are very common among young people, with two-thirds of new chlamydia and half of gonorrhea infections occurring among people between 15 and 24 years of age. It has been estimated that 1 in 20 sexually active women aged 14-24 has chlamydia.5
Figure 1. Weekly Reported U.S. STI Cases: 2020 vs 2019
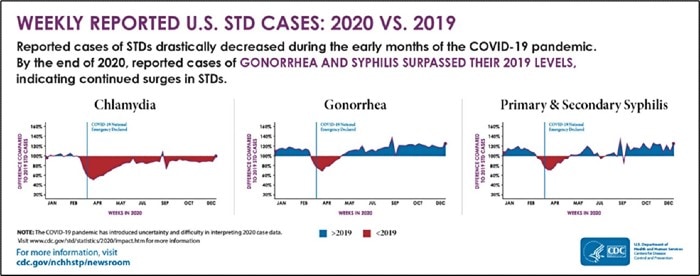
Figure 2. Costs Associated with New STIs in a Single Year
ASYMPTOMATIC INFECTIONS ARE COMMON; MORE SCREENING IS NEEDED
Chlamydial infections are asymptomatic in 70% to 75% of women,6 while gonorrhea is asymptomatic in 50% of women.7 This high prevalence of asymptomatic infections led the CDC to recommend routine chlamydia and gonorrhea screening for all sexually active women aged 15-24, as this population accounts for almost half of all new STD infections yearly.6 (See Table 1.) Despite public health efforts, current data suggest that only a very small percentage of adolescent women are screened annually. A national survey of youth 15–25 years of age found that most had never received an STI test; only 16.6% of females and 6.6% of males had been tested in the past 12 months.8
| Table 1. CDC STD Screening Guidelines for Chlamydia and Gonorrhea6 |
| Routine screening for chlamydia and gonorrhea infection on an annual basis is recommended for all sexually active females aged <25 yearsWomen >25 years who have a new sex partner in the last year, have more than one sex partner, 25 yearsScreening of sexually active young men should be considered in clinical settings with a high prevalence of chlamydiaMore frequent screening than annual for certain women (eg, adolescents) or certain men (eg, men who have sex with men) might be indicated on the basis of risk behaviors.All persons diagnosed with gonorrhea or chlamydia should be rescreened 3 months after treatment. |
A Role for Urgent Care
In the 1980s and 1990s, most STI care was provided in dedicated STI clinics. Funding cuts at the beginning of this century have led to a decrease in these specialty clinics, with almost 80% of STI cases now diagnosed in non-STI clinics.9 This closure of STI clinics, along with generational changes in how younger people access healthcare, has increased the utilization of urgent care for STI diagnosis and treatment. Currently, only 55% of Generation Z (those aged 10-25 in 2022) report having a primary care doctor. Millennials (those aged 26-41 in 2022) have a slightly higher rate of an identified PCP (65%), but 24% of them admit it has been 5 or more years since their last annual exam.10
The lack of an established provider-patient relationship contributes to adolescents having greater concerns about confidentiality and makes them less likely than adults to utilize sexual health services. This reluctance to seek timely care leads to lower rates of diagnosis and treatment in this age group, which in turn causes higher rates of disease transmission.11
This is concerning because C trachomatis (CT) and N gonorrhoeae (NG) infections can lead to multiple sequelae (which are exacerbated by delayed diagnosis and treatment), the most serious of which include:
- Pelvic inflammatory disease
- Ectopic pregnancy
- Infertility
- Chronic pelvic pain
- Increased risk for HIV transmission and acquisition
Because so many infections are initially asymptomatic, some women don’t exhibit recognizable symptoms until complications (eg, PID) have occurred.
2021 CDC STI TREATMENT GUIDELINES FOR CT, NG, AND TV
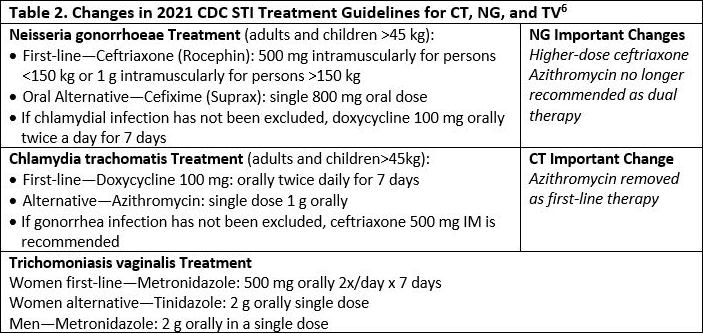
The 2021 CDC STD Treatment Guidelines6 contain several important changes from the previous guidelines, released in 2015. See Table 2.
Presumptive treatment with antimicrobials for C trachomatis and N gonorrhoeae should be provided for women at increased risk (eg, those aged <25 years and women with a new sex partner, a sex partner with concurrent partners, or a sex partner who has an STI), if follow-up cannot be ensured, or if testing with NAAT is not possible.6
Rationale for Guideline Changes
A global surge of antibiotic resistance among bacterial STIs, especially gonorrhea, has left ceftriaxone as the single reliable drug option for gonorrhea. The change to a higher dose of ceftriaxone was based on new pharmacokinetic and pharmacodynamic modeling that revealed 250 mg ceftriaxone has insufficient MIC to adequately treat gonorrhea in all cases. It was also determined that an even higher dose was necessary for patients >150 kg.
The removal of azithromycin as dual therapy for gonorrhea was based in part on the CDC’s Gonococcal Isolate Surveillance Project (GISP), which found the percentage of gonorrhea isolates exhibiting resistance to azithromycin increased more than seven-fold from 2013 to 2018.12 The removal of azithromycin as a first-line treatment for chlamydia was based on recent evidence that raised concern for its efficacy, especially in rectal infections.13

TO TREAT OR NOT TO TREAT? KEY CHALLENGES WITH STD TESTING TODAY
Common STD symptoms, such as dysuria, lower abdominal pain, vaginal discharge, and testicular pain, are often not specific enough to make a definitive diagnosis, which makes diagnostic testing a necessity. CT and NG are commonly tested for at the same time due to similar symptoms and the prevalence of co-infections. The problem in urgent care is that the vast majority of current, routine STI testing must be sent out to a reference lab, with results often unavailable for 2-4 days or longer. Delaysin testing for STIs require urgent care providers to make treatment decisions without the benefit of test results; this poses significant challenges for patients and providers, along with the potential to disrupt the clinic’s workflow.
Challenges for patients include:
- Overtreatment: Patients treated presumptively with antibiotics who then test negative are placed at risk for antibiotic complications unnecessarily
- Undertreatment: Patients who are not presumptively treated with antibiotics, but who then test positive, can have disease progression and further transmit disease
- Undertreated patients may be lost to follow up. A recent study found that 20% of patients with positive CT and NG screening cultures did not return to the clinic within 30 days for treatment. Further, of those who did return, 30% did so only after 14 days.14
- Missed opportunity for expedited partner(s) treatment (EPT) at initial visit
- Lack of a definitive diagnosis creates a missed opportunity for patient education
Providers, on the other hand, may be forced to make treatment decisions without benefit of test results. These include:
- Overtreatment: Prescribing unnecessary antibiotics which may contribute to antibiotic resistance
- Provider continuity: New providers must review the patient’s medical record before making follow-up and treatment decisions
Finally, the clinic workflow may be disrupted in the following ways:
- Time burden and cost of delayed results notification and follow up scheduling—eg, gonorrhea-positive patients (not treated presumptively) will need to return to the clinic for CDC-recommended first-line treatment (IM ceftriaxone)
- Relaying positive STI testing results over the phone is not ideal for patient or provider
- Patients lost to follow-up
A New Era in STD Testing
The disadvantages of the prolonged turnaround time of CT and NG test results have not gone unrecognized by researchers and public health officials. In 2016, the World Health Organization developed the Sustainable Development Goal of ending STI epidemics as major public health threats by 2030.15 In response to the WHO goal, the National Institutes of Health has declared there is a critical need for the development of innovative, rapid, point-of-care diagnostic tests, new treatments, and vaccines for STIs.16
Several recent studies have demonstrated improvements in both over- and undertreatment of CT and NG when using rapid molecular POC tests vs “send-out” nucleic acid amplification tests (NAAT).
May, et al, found a significant reduction in unnecessary antibiotic treatment (overtreatment) for CT/NG in subjects tested on a rapid molecular test compared with those tested with delayed NAAT.17 Gaydos, et al,18 also compared rapid CT/NG testing vs delayed CT and NG testing. In this study, none of the patients in the rapid testing group were undertreated compared with 56% who were undertreated in the routine testing group. Overtreatment was reduced, as well, with only 25% unnecessarily treated in the rapid testing group vs 47% in the routine testing group.
Help appears to be on the way, as indicated by the recent clinical trial results of a rapid PCR test for CT, NG, and TV in women, which were published in Lancet Infectious Diseases (2021). The device had a sensitivity of 97.6% and specificity of 98.3% for chlamydia and a sensitivity of 97.4% and specificity of 99.4% for gonorrhea.19 It received FDA clearance in late 2021 as a point-of-care test and was also granted a CLIA Waiver, making it accessible for use in urgent care .
The availability of accurate and rapid molecular POC testing for STIs could help solve many of the challenges that delayed testing creates, including the following:
- Significantly reduce undertreatment and the long-term consequences of untreated STIs
- Reduce presumptive overtreatment with unnecessary antibiotics
- Prompter treatment for positive patients, face-to-face educational window, and quicker opportunity for EPT
- Reduce loss of patient follow-up and negate need for an additional visit specifically for test results or IM treatment
- Reduce chances of ongoing community transmission
- Reduced staff time spent contacting patients to inform them of send-out lab results and scheduling repeat visit if treatment needed
DISCUSSION
Young people 15 to 24 years of age are most at risk from the STI epidemic. Because so many CT and NG infections are asymptomatic, screening should be performed on at least an annual basis for sexually active women in this age group. However, many young people do not have annual exams or PCPs, and current U.S. screening rates hover below 50%.20 Unfortunately, readily available low-cost antigen- and antibody-detection POC tests for CT and NG are insufficiently sensitive for screening purposes.16 The recent FDA approval of accurate, rapid, molecular POC testing for CT and NG with sufficient sensitivity for screening makes urgent care clinics well-positioned to become a solution to this problem.
Unfortunately, there is an elephant in the room: While molecular POC CT NG tests are much more sensitive and are better suited to screening, they are also more expensive. This creates a significant barrier to adoption for clinics with flat-fee payer contracts.. In this type of payer arrangement, the urgent care clinic is paid a specified dollar amount for each of the payer’s patients they care for. If the clinic wishes to use a faster, more accurate molecular POC CT NG test they must bear 100% of the additional cost burden of purchasing and performing the test while receiving no additional reimbursement.
A very significant percentage of UCCs have flat-fee payer contracts. As such, their patients may be unlikely to take advantage of this new technology that has the potential to enable a seismic shift in our approach to STI treatment and prevention in urgent care. The urgent care community’s ability to participate in and bolster public health testing and screening initiatives against the STI epidemic could be substantially dampened if this remains the case. Guidance from health insurers on what clinical data and economic endpoints would support the use of molecular POC molecular tests in our setting would benefit urgent care researchers.
CONCLUSION
The STI epidemic emerged during an era of increasing bacterial resistance.21 Neisseria gonorrhea resistance is of particular concern, with cephalosporins the only remaining drug class still effective for treatment. New CDC gonorrhea treatment guidelines now recommend a higher dose of ceftriaxone, and the removal of azithromycin as a first-line treatment. Doxycycline remains the drug of choice for chlamydia, with azithromycin now listed as an alternative therapy due to increased resistance.
The CDC estimates that between 30% and 50% of antibiotics prescribed in outpatient clinics are unnecessary.22 They strongly encourage antibiotic monotherapy to treat gonorrhea and chlamydia when test results are known at the time of treatment decision. New rapid molecular POC testing devices, if economically feasible, will give urgent care providers the ability to make earlier and more informed treatment decisions, as well as better steward their antibiotic use by prescribing monotherapy for CT and NG when results indicated. Recent studies have shown that these tests also have the potential to significantly reduce over- and undertreatment with antibiotics.
Rates of gonorrhea and chlamydia continue to rise, and our young people aged 15-24 are most at risk from this epidemic. Because so many CT and NG infections are asymptomatic, annual screening in this age group is essential to stem the tide of STIs. Urgent care is uniquely positioned to address the current gaps in screening. We should embrace the opportunity and responsibility of providing screening services to this vulnerable population.
REFERENCES
- 2020 STD Surveillance Report Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Accessed 6/16/2022
- 2019 STD Surveillance Report Division of STD Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Accessed 6/16/2022
- Chesson, Harrell W. PhD; Spicknall, Ian H. PhD; Bingham, Adrienna PhD; Brisson, Marc PhD; Eppink, Samuel T. PhD; Farnham, Paul G. PhD; Kreisel, Kristen M. PhD; Kumar, Sagar MPH,∥; Laprise, Jean-François PhD; Peterman, Thomas A. MD; Roberts, Henry PhD; Gift, Thomas L. PhD The Estimated Direct Lifetime Medical Costs of Sexually Transmitted Infections Acquired in the United States in 2018, Sexually Transmitted Diseases: April 2021 – Volume 48 – Issue 4 – p 215-221 doi: 10.1097/OLQ.0000000000001380
- Mayer KH, de Vries H. HIV and sexually transmitted infections: Responding to the “newest normal”. J Int AIDS Soc 2018; 21:e25164.
- Kreisel KM, Spicknall IH, Gargano JW, Lewis FM, Lewis RM, Markowitz LE, Roberts H, Satcher Johnson A, Song R, St. Cyr SB, Weston EJ, Torrone EA, Weinstock HS. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2018. Sex Transm Dis 2021
- Workowski KA et al. Sexually transmitted infections treatment guidelines. MMWR – Recommendations and Reports. 2021;70(4):1
- Workowski KA. Centers for Disease Control and Prevention sexually transmitted diseases treatment guidelines. Clinical Infectious Diseases. 2015;61(8):S759-S762
- Cuffe KM, et al. Sexually transmitted infection testing among adolescents and young adults in the United States. Journal of Adolescent Health. 2016;58(5):512–519.
- Barrow RY, Ahmed F, Bolan GA, Workowski KA. Recommendations for Providing Quality Sexually Transmitted Diseases Clinical Services, 2020. MMWR Recomm Rep 2020;68(No. RR-5):1–20. DOI: http://dx.doi.org/10.15585/mmwr.rr6805a1
- Employee Benefit Research Institute. Attitudes toward primary care providers differ by generation. Available at: https://www.ebri.org/docs/default-source/infographics/46_ig-cehcs2-6feb20.pdf?sfvrsn=64793d2f_4. February 6, 2020
- American College of Obstetricians and Gynecologists. Spotlight on Chlamydia: Annual Screenings a Must for Young Women. San Diego, CA: Obstetrics & Gynecology, 2007
- CDC. Sexually transmitted disease surveillance 2019 [Internet]. Atlanta GA: US Department of Health and Human Services, CDC; 2021. https://www.cdc.gov/std/statistics/2019/default.htm
- Kong FY, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014;59:193–205. 10.1093/cid/ciu220
- Schwebke, Jane R. MD†; Sadler, Robert†; Sutton, Michael J. Ba†; Hook, Edward W. Iii MD† Positive Screening Tests for Gonorrhea and Chlamydial Infection Fail to Lead Consistently to Treatment of Patients Attending a Sexually Transmitted Disease Clinic, Sexually Transmitted Diseases: April 1997 – Volume 24 – Issue 4 – p 181-184
- World Health Organization. Global health sector strategy on sexually transmitted infections 2016–2021: towards ending STIs. Geneva, Switzerland: WHO, 2016.
- Eisinger RW, Erbelding E, Fauci AS. Refocusing Research on Sexually Transmitted Infections. J Infect Dis. 2020 Oct 1;222(9):1432-1434. doi: 10.1093/infdis/jiz442. PMID: 31495889; PMCID: PMC7529043
- May, Larissa MD; Ware, Chelsea E. MS; Jordan, Jeanne A. PhD; Zocchi, Mark MPH; Zatorski, Catherine BA; Ajabnoor, Yasser MD; Pines, Jesse M. MD A Randomized Controlled Trial Comparing the Treatment of Patients Tested for Chlamydia and Gonorrhea After a Rapid Polymerase Chain Reaction Test Versus Standard of Care Testing, Sexually Transmitted Diseases: May 2016 – Volume 43 – Issue 5 – p 290-295 doi: 10.1097/OLQ.0000000000000438
- Gaydos CA, Ako MC, Lewis M, Hsieh YH, Rothman RE, Dugas AF. Use of a Rapid Diagnostic for Chlamydia trachomatis and Neisseria gonorrhoeae for Women in the Emergency Department Can Improve Clinical Management: Report of a Randomized Clinical Trial. Ann Emerg Med. 2019 Jul;74(1):36-44. doi: 10.1016/j.annemergmed.2018.09.012. Epub 2018 Nov 2. PMID: 30392736; PMCID: PMC6494710
- Morris SR, Bristow CC, Wierzbicki MR, et al. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis. 2021;21:668-676
- Morbidity and Mortality Weekly Report (MMWR) Chlamydia Screening Among Females Aged 15–21 Years — Multiple Data Sources, United States, 1999–2010 Supplements September 12, 2014 / 63(02);80-88
- Morehead MS, Scarbrough C. Emergence of Global Antibiotic Resistance. Prim Care. 2018; 45(3):467–84.
Manuscript submitted August 15, 2022; accepted October 14, 2022.
Author affiliations: Glenn Harnett, MD, No Resistance Consulting Group; JUCM Editorial Board. In the past 12 months Dr. Harnett has received consulting fees from Visby Medical’s marketing and commercial department for hosting an educational webinar, organizing a marketing focus group, and presenting a seminar on the urgent care industry to the management team. He is an active principal investigator in Vi+sby clinical trials for respiratory and STI indications.

