Urgent Message: Mycoplasma species, most notably Mycoplasma genitalium are an increasingly recognized etiology of persistent urethral discomfort and non-gonococcal urethritis in males. These infections are commonly asymptomatic and can also have significant consequences in females. Antibiotic resistance is a common concern and treatment recommendations differ significantly from those for other sexually transmitted infections.
Joseph Something, PA-C; Greg Smithers, PA-C; Ina Park, MD, MS
Citation: Something J, Smithers G, Park I. A Review of Genital Mycoplasma and Ureaplasma Infections for the Urgent Care Clinician. J Urgent Care Med. 2024; 18(8):13-19
Clinical Scenario
A 23-year-old male presented to urgent care (UC) with 5 weeks of persistent, mild, non-purulent penile discharge and urethral discomfort. He reported having condomless sex with both male and female partners in the prior 6 months since he last had sexually transmitted infection (STI) testing. He was seen 2 weeks prior with a urine dip showing trace leukocyte esterase (LE), a negative polymerase chain reaction (PCR) test for gonorrhea (GC) and chlamydia (CT) and a negative urine culture. At the second visit, his genitourinary (GU) exam revealed no scrotal swelling or tenderness and scant, mucoid urethral discharge. The patient subsequently underwent urine PCR testing for Mycoplasma genitalium, which was positive, and he was treated with 7 days of doxycycline followed by 7 days of moxifloxacin.
Introduction and Background
Mycoplasma is a genus of atypical bacteria within the class Mollicutes. There are over 200 named Mycoplasma species, but only a limited number of which have been identified in the human genitourinary tract: Mycoplasma genitalium (Mgen), M. hominis, M. fermentans, M. penetrans, Ureaplasma urealyticum, U parvum.[1],[2] Note that despite the difference in genus name, Ureaplasma organisms are also Mycoplasma species.
Mycoplasma spp. have proven to be difficult organisms to study due to the frequency of asymptomatic carriage, co-infection with other urogenital pathogens that cause similar symptoms, design limitations in studies performed, and difficulty in culturing the organisms.1
Although the presence of the 6 Mycoplasma spp. mentioned above are frequently asymptomatic, any of them can cause a variety of symptomatic infections and complications ranging from pneumonia, spontaneous abortion, infectious arthritis, meningitis, bacteriemia, and various neonatal complications such as preterm birth and bronchopulmonary disease.1,[3],[4],[5] Asymptomatic urogenital colonization is common but may also result in symptomatic urethritis and epididymitis in males and cervicitis, pelvic inflammatory disease (PID), and infertility in females.[6]
This review focuses on Mgen, given that it is the most likely Mycoplasma spp. to result in symptomatic urogenital disease, however, other organisms in this class may be identified incidentally when testing for a cause of unexplained urogenital symptoms.
Prevalence
According to a 2018 systematic review and meta-analysis, the prevalence of Mgen in the general population was 1.3% in higher-income countries and 3.9% in low- and middle-income countries. Prevalence was similar between males and females. In asymptomatic patients, the prevalence across studies was 0.8%. Additionally, prevalence in specific groups showed rates of 0.9% among pregnant people, 3.2% among males who have sex with males (MSM), and 15.9% among female commercial sex workers (CSWs).[7] Co-infection with other STIs such as GC, CT, and human immunodeficiency virus (HIV) have also been shown to correlate with an increased prevalence of Mgen.[8],[9],[10]
The rates of adult urogenital mycoplasma spp. colonization is between 1-2% with increased rates among people with more frequent condomless sex and/or higher numbers of total sexual partners.9,[11] MSM who are HIV-positive also have significant risk of Mgen rectal colonization, however, they commonly are asymptomatic.10,[12]
Modes of Transmission
The primary mode of transmission for Mgen is through direct mucosal contact during vaginal or anal sex.[13] Outside of urogenital symptoms, rectal colonization is similarly common compared to urogenital colonization, however, anorectal symptoms are less likely to occur than with urogenital infections.8 Although studies are limited, Mgen appears to be dissimilar from other STIs in that it does not appear to be easily transferred and/or cause symptoms with oral-genital contact.[14],[15] Data are sparse on the efficacy of condoms at prevention of Mgen, however barrier use is still recommended as a means of prevention.3,8
Clinical Presentation
The incubation period of Mgen is estimated to vary from 4-8 weeks after exposure. This extended incubation period relative to other bacterial urogenital pathogens (eg, GC, CT) is attributed to the slow-growth and reproductive rate of mycoplasma.[16] Penile or vaginal discharge and urethral pain are common symptoms, but infections have been reported to be asymptomatic in up to 90% of cases in males and in 60% of cases in females.[17]
Up to 35% of cases of non-gonococcal urethritis (NGU) in males and 10-25% of cases of cervicitis/PID in females are attributable to Mgen. Other common symptoms and presentations in males include dysuria, urethral discharge, proctitis, chronic urethritis, and/or balanoposthitis. Females with symptomatic Mgen infections present most commonly with dysuria, urgency, lower abdominal pain, post-coital bleeding, or menorrhagia.3
Although rectal colonization may occur in males and females, Mgen proctitis appears to be clinically rare in females.8,[18],[19]
Diagnostic Testing
When testing is performed for Mycoplasma spp., a nucleic acid amplification test, such as a PCR, is preferred. Samples can be obtained by first-void or first-catch urine from males, vaginal swabs from females, and rectal swabs. Self-collected vaginal swabs areas accurate as clinician-obtained endocervical swabs.[20] Anorectal swabs should be collected only when a patient presents with symptoms of proctitis and exclusion of GC and CT has been confirmed.3 Other tests, such as culture and antigen, are not recommended as they have poor test characteristics and results may take an impractically long time to return. Although not often available in the US, macrolide-susceptibility testing is highly recommended where available.3,8
Testing Recommendations
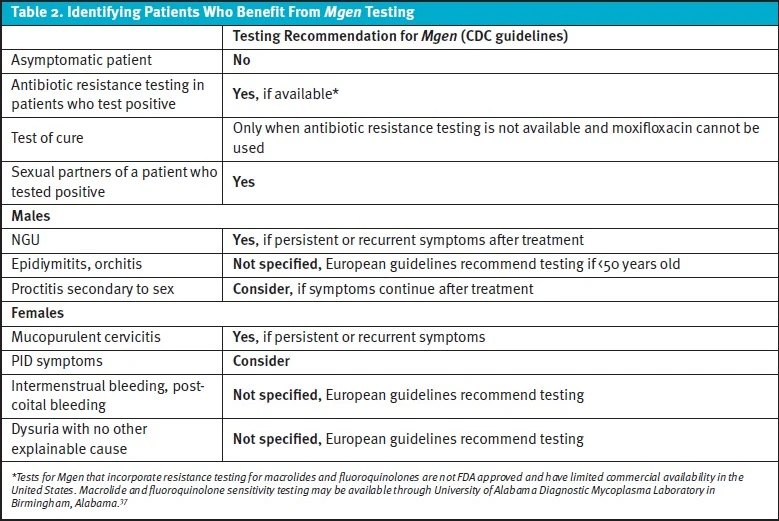
The Centers for Disease Control and Prevention (CDC) does not recommend routine or asymptomatic testing for Mgen for any population, but does recommend in males with recurrent NGU and in females who have recurrent cervicitis or at initial presentation of PID. In cases where GC and CT have been ruled-out but Mgen testing is unavailable, the CDC recommends empiric treatment should be offered for patients with symptoms of persistent/recurrent urethritis or cervicitis and considered for patients with PID.8 Partners of patients with Mgen should be offered testing and treated if their test results are positive. Expedited partner therapy is not recommended.
The most recent Journal of the European Academy of Dermatology and Venerology (JEADV) recommendations are more comprehensive. These European guidelines recommend both testing and treatment if a patient has had genital sexual contact with a partner who has tested positive for Mgen but has not been treated. Testing should also be performed for anyone who has ongoing sexual contact with individuals treated for Mgen. Testing should be considered for anyone with proctitis after GC and CT have been excluded.3
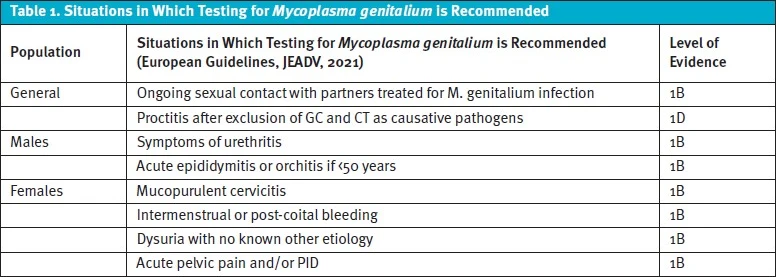
Testing is recommended in males presenting with signs/symptoms of urethritis or if a male below the age of 50 is experiencing acute epididymitis/orchitis, especially in cases with negative GC/CT testing and risk factors for infection by sexual history. Similarly, females should be tested if they exhibit any of the following: mucopurulent cervicitis, intermenstrual or post-coital bleeding, dysuria with no known other etiology, acute pelvic pain, and/or PID.3 The European recommendations and their corresponding levels of evidence are summarized in Table 1.
Treatment Recommendations for Mgen in Adults and Adolescents
Mgen can be a challenging infection to eradicate. As such, treatment recommendations differ significantly from those for other causes of urethritis (ie, CT, GC). For example, treatment for one week with doxycycline alone has only a <40% cure rate when Mgen infection is present.
In a recent study, rates of macrolide resistance mutations in Mgen samples ranged from 51-71% in 6 US STI clinics.[21] Given high levels of resistance, macrolide-resistance testing (when available) is recommended by both the CDC and European guidelines (although none of these assays are currently approved by the US Food and Drug Administration [FDA]).3,8,[22] If Mgen is detected, initial treatment recommendations suggest beginning with doxycycline 100mg twice daily for 7 days followed by either azithromycin (if strain is known to be sensitive to macrolides) or moxifloxacin for 7 days if resistance testing was not performed (see Algorithim 1).8 As macrolide-susceptibility testing is not available in most UC settings, the 2-stage treatment with doxycycline and moxifloxacin will likely be the standard protocol.
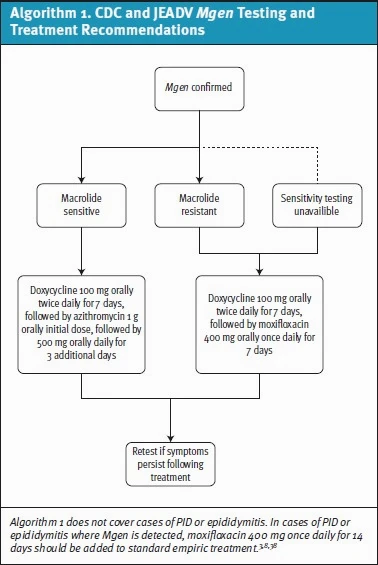
Moxifloxacin Safety Considerations
Fluoroquinolones have been associated with tendonitis and tendon rupture, which can occur early during the course of therapy or after completion. Compared to ciprofloxacin and levofloxacin, moxifloxacin has a weaker association with tendonitis and tendon rupture, however an analysis of the FDA Adverse Events Reporting System reported a significant association for all three fluoroquinolones and these outcomes. Patients should be cautioned about this association and stop therapy if symptoms of tendinopathy develop during therapy.[23]
With use of moxifloxacin, weighing the possible cardiac side effects is important, as moxifloxacin is associated with the highest risk of QTc prolongation among the fluoroquinolones.3,8,[24] Before prescribing moxifloxacin, reviewing the patient’s other medications and holding any non-essential medication that may affect QTc during therapy is prudent. There are no formal recommendations for monitoring, however, in uncertain cases, a baseline electrocardiogram may be helpful in ensuring the patient’s QTc is not prolonged before initiating therapy. As most patients with fluoroquinolone-associated torsades de pointes (TdP) and sudden death had borderline or prolonged baseline QTc, caution is advised in such cases if prescribing moxifloxacin.24,[25]
Treatment Recommendations in Pregnant Patients
Mgen infections during pregnancy have been shown to slightly increase the risk of spontaneous abortion and preterm birth. In pregnant patients, the recommended first-line treatment is for an immediate 5-day course of azithromycin during pregnancy or otherwise postponed until after pregnancy, according to JEADV. However, as many strains of Mgen are macrolide-resistant, regardless if azithromycin is prescribed from UC, follow-up and ongoing discussion with the patient’s obstetric clinician is critical. Little is known about the likelihood of Mgen transmission during birth and risks of neonatal infections such as conjunctivitis and/or respiratory tract infection.3

The CDC does not have specific recommendations for treatment during pregnancy, instead, the CDC recommends contacting STD Clinical Consultation Network (https://www.stdccn.org/render/Public) to discuss pregnant patients further.
Follow-up and Referral Recommendations
Unfortunately, the CDC guidelines do not offer much guidance regarding partner testing and treatment. This is primarily due to the limitations of the existing evidence. The European guidelines outlined in the JEADV do recommend that partners of patients infected with Mgen should be tested and treated if positive for infection.3 Initiation of treatment for a patient with a known exposure is reasonable while awaiting PCR test results. Additionally, if there is a strong concern for partner infection and testing is not possible, EPT is appropriate.
Patients should be advised to abstain from sexual activity until after completion of treatment and symptoms have entirely resolved. Test-of-cure is not indicated in patients in whom symptoms have resolved and who received treatment; like in GC and CT infections, molecular retesting for cure may yield a false positive in the weeks following treatment due to residual genetic material.8

While uncommon, if clinical failure following treatment with both doxycycline and moxifloaxcin occurs referral or consultation with an infectious disease expert in sexually transmitted infections is advisable.[26] In the US, questions can be directed to the STD Clinical Consultation Network (https://www.stdccn.org/render/Public).
Ureaplasma and Other Mycoplasma spp.
Like the Mycoplasma spp., Ureaplasma spp., specifically U. urealyticum and U. parvum., are a class of mollicutes that are commonly identified in the GU tract of both males and females. While FDA-approved assays test exclusively for Mycoplasma, multiple laboratories offer internally developed assays that test for both Mycoplasma spp. and Ureaplasma spp. with a single sample. The CDC specifically does not recommend testing for Ureaplasma spp.[27],[28] While Ureaplasma are often considered normal genital flora, the presence of the organism has been associated with various GU conditions as well, making defining and managing Ureaplasma a clinical quandary. Notably, their presence have been associated with varying symptomatic GU diseases conditions such as bacterial vaginosis (BV), PID, and most commonly urethritis in males.[29],[30]
Ureaplasma is not discussed extensively in this review given the lack of clear guidelines for testing for Ureaplasma spp. Additionally, antibiotic resistance of Ureaplasma is fairly uncommon compared to Mycoplasma. A recent study in the United States reported resistance in only 1 of 202 U. parvum isolates.30,[31] As such, doxycycline 100mg twice daily for 7 days is usually adequate for treating symptomatic patients with Ureaplasma. For patients with persistent symptoms after completing 7 days of doxycycline, some experts recommend to proceed with azithromycin 1g orally followed by 500mg once daily for 2 days plus metronidazole 400mg twice daily for 5 days.31 As with Mgen, there is little high-quality guidance regarding partner testing and treatment. Current recommendations do suggest partners should be tested and then treated if infection is detected.31
M. hominins has received even less attention compared to other species of Mycoplasma. However, like Ureaplasma spp, M. hominins is generally considered normal genital flora, but vexingly has also been associated with varying GU conditions including cystitis, BV, PID.[32] There is no compelling evidence suggesting causation of urethritis by M. hominis.
It has been suggested that M. hominis acts symbiotically with other organisms that contribute to clinical BV. Still there are conflicting opinions on the significance of how it may affect the course of BV and causality has not been established.32,[33] There are no guidelines for differentiating treatment or testing for M. hominins from other Mycoplasma and Ureaplasma spp.33 M hominis is typically susceptible in vitro to tetracycline, clindamycin, and fluoroquinilones.32,33. Therefore, treatment regimens for Ureaplasma GU infections would likely cover for M. hominis as well.
Immunocompromised adults are prone to M. hominis infections including septic arthritis, prosthetic joint infection, central nervous system infections, and infective endocarditis.32 Neonatal infections increase risk for prematurity, low birth weight, pneumonia, bacteremia, meningitis, and chronic lung disease.32
The role of M. fermentans and M. penetrans in human disease are controversial. M. fementans has been identified in the GU tract but studies have failed to confirm a clear association of M. fermentans with GU pathology. Limited evidence suggests that M. fermentans may have a contributory role in a variety of idiopathic conditions ranging from autoimmune disease to chronic fatigue syndrome.[34],[35],[36] M. penetrans has not generally been associated with symptomatic GU infections either, but the prevalence does seem to be increased in HIV-positive patients and MSM populations for uncertain reasons. Further research is needed to clarify the role of these non-Mgen Mycoplasma in the etiologies of various disease states, but testing for these Mycoplasma spp. is generally limited to research settings at this time.2,36
Takeaway Points
- Mycoplasma and Ureaplasma species can produce a variety of GU symptoms in males and females.
- Mgen is a STI and, while frequently asymptomatic, is a common cause of NGU.
- CSW, MSM, and those infected with other STIs are at the highest risk for Mgen.
- The incubation period for Mycoplasma infections is 1-2 months and both GU and anorectal infection should be considered. Mycoplasma does not appear to be a significant pathogen in the oropharynx.
- The most recent guidelines from the US and Europe recommend testing in patients with suggestive symptoms who fail empiric therapy and have negative GC/CT testing.
- Given rising rates of macrolide resistance, recommended treatment involves 7 days of doxycycline followed by 7 days of moxifloxacin (unless azithromycin sensitivity can be confirmed).
- Antibiotic selection and treatment duration differ based on site of infection (eg, urethritis vs proctitis vs PID) and also for pregnant patients.
- Standard treatment includes using moxifloxacin which can have dangerous side-effects including TdP. Consider the patient’s overall health carefully before treating.
- Patients should be counseled to abstain from sexual contact until asymptomatic and appropriate therapy has been completed.
Manuscript submitted March 13, 2024; accepted April 2, 2024.
Author Affiliations: Joseph Something, PA-C, Legacy/GoHealth, Portland, Oregon. Greg Smithers, PA-C, Legacy/GoHealth, Portland, Oregon. Ina Park, MD, MS, California Prevention Training Center, Department of Family and Community Medicine at University of California San Francisco, California.
References
- [1]. Combaz-Söhnchen, N., Kuhn, A. (2017). A Systematic Review of Mycoplasma and Ureaplasma in Urogynaecology. Geburtshilfe und Frauenheilkunde, 77(12), 1299-1303. https://doi.org/10.1055/s-0043-119687
- [2]. Gardette M, Touati A, Laurier-Nadalié C, Bébéar C, Pereyre S. Prevalence of Mycoplasma penetrans in Urogenital Samples From Men Screened for Bacterial Sexually Transmitted Infections. Open Forum Infect Dis. 2023 Apr 4;10(4):ofad180. doi: 10.1093/ofid/ofad180. PMID: 37082616; PMCID: PMC10111059.
- [3]. Jensen JS, Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2022 May;36(5):641-650. doi: 10.1111/jdv.17972. Epub 2022 Feb 19. PMID: 35182080.
- 4]. Frenzer C, Egli-Gany D, Vallely LM, Vallely AJ, Low N. Adverse pregnancy and perinatal outcomes associated with Mycoplasma genitalium: systematic review and meta-analysis. Sex Transm Infect. 2022 May;98(3):222-227. doi: 10.1136/sextrans-2021-055352. Epub 2022 Mar 29. PMID: 35351816; PMCID: PMC9016252.
- [5]. Garland SM, Kelly VN. Role of genital mycoplasmas in bacteremia: should we be routinely culturing for these organisms? Infect Dis Obstet Gynecol. 1996;4(6):329-32. doi: 10.1155/S106474499600066X. PMID: 18476120; PMCID: PMC2364519.
- [6]. Rajkumari N, Kaur H, Roy A, Gupta N, Dhaliwal LK, Sethi S. Association of Mycoplasma genitalium with infertility in North Indian women. Indian J Sex Transm Dis AIDS. 2015 Jul-Dec;36(2):144-8. doi: 10.4103/0253-7184.167141. PMID: 26692605; PMCID: PMC4660553.
- [7]. Baumann L, Cina M, Egli-Gany D, et al. Prevalence of Mycoplasma genitalium in different population groups: systematic review and meta-analysis. Sexually Transmitted Infections 2018;94:255-262.
- [8]. Centers for Disease Control and Prevention. Mycoplasma genitalium – STI Treatment Guidelines. Published July 14, 2021. Accessed from www.cdc.gov.
- [9]. Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. Mycoplasma genitalium compared to chlamydia, gonorrhoea and trichomonas as an aetiological agent of urethritis in men attending STD clinics. Sex Transm Infect. 2009;85:438–440. doi: 10.1136/sti.2008.035477.
- [10]. Falk L, Fredlund H, Jensen JS. Symptomatic urethritis is more prevalent in men infected with Mycoplasma genitalium than with Chlamydia trachomatis. Sex Transm Infect. 2004;80:289–293. doi: 10.1136/sti.2003.006817.
- [11]. Torrone EA, Kruszon-Moran D, Philips C, et al. Prevalence of Urogenital Mycoplasma genitalium Infection, United States, 2017 to 2018. Sex Transm Dis. 2021;48(11):e160-e162. doi:10.1097/OLQ.0000000000001394
- [12]. Leung A, Eastick K, Haddon L, Horn K, Ahuja D, Horner P. Mycoplasma genitalium is associated with symptomatic urethritis. Int J STD AIDS. 2006;17:285–288. doi: 10.1258/095646206776790231.
- [13]. Ma L., Taylor S., Jensen J.S., et al. Short tandem repeat sequences in the Mycoplasma genitaliumgenome and their use in a multilocus genotyping system. BMC Microbiol. 8, 130 (2008). https://doi.org/10.1186/1471-2180-8-130
- [14]. Bradshaw CS, Fairley CK, Lister NA, Chen SJ, Garland SM, Tabrizi SN. Mycoplasma genitalium in men who have sex with men at male-only saunas. Sex Transm Infect 2009; 85: 432–435.
- [15. Latimer RL, Vodstrcil L, De Petra V et al. Extragenital Mycoplasma genitalium infections among men who have sex with men. Sex Transm Infect 2020; 96: 10–18.
- [16]. Seña AC, Lee JY, Schwebke J, et al. A Silent Epidemic: The Prevalence, Incidence and Persistence of Mycoplasma genitalium Among Young, Asymptomatic High-Risk Women in the United States. Clin Infect Dis. 2018;67(1):73-79. doi:10.1093/cid/ciy025.
- [17]. Sonnenberg P, et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Int J Epidemiol. 2015 Dec;44(6):1982-94. doi: 10.1093/ije/dyv194. PMID: 26534946; PMCID: PMC4690003.
- [18]. Han Y, Yin YP, Liu JW, Chen K, Zhu BY, Zhou K, Shi MQ, Xu WQ, Jhaveri TA, Chen XS. Rectal Mycoplasma genitalium in Patients Attending Sexually Transmitted Disease Clinics in China: An Infection That Cannot Be Ignored. Infect Drug Resist. 2021 Jun 30;14:2509-2515. doi: 10.2147/IDR.S314775. PMID: 34234478; PMCID: PMC8255646.
- [19]. Khosropour CM, Jensen JS, Soge OO, Leipertz G, Unutzer A, Pascual R, Barbee LA, Dombrowski JC, Golden MR, Manhart LE. High Prevalence of Vaginal and Rectal Mycoplasma genitalium Macrolide Resistance Among Female Sexually Transmitted Disease Clinic Patients in Seattle, Washington. Sex Transm Dis. 2020 May;47(5):321-325. doi: 10.1097/OLQ.0000000000001148.
- [20]. Lillis, R. A., Nsuami, M. J., Myers, L., & Martin, D. H. (2011). Utility of urine, vaginal, cervical, and rectal specimens for detection of Mycoplasma genitalium in women. Journal of clinical microbiology, 49(5), 1990-1992.
- [21]. Manhart LE, Leipertz G, Soge OO, Jordan SJ, McNeil C, Pathela P, Reno H, Wendel K, Parker A, Geisler WM, Getman D, Golden MR; MyGeniUS Study Team. Mycoplasma genitalium in the US (MyGeniUS): Surveillance Data From Sexual Health Clinics in 4 US Regions. Clin Infect Dis. 2023 Nov 17;77(10):1449-1459. doi: 10.1093/cid/ciad405. PMID: 37402645.
- [22]. Mitjà O, Suñer C, Giacani L, Vall-Mayans M, Tiplica GS, Ross JDC, Bradshaw CS. Treatment of bacterial sexually transmitted infections in Europe: gonorrhoea, Mycoplasma genitalium, and syphilis. Lancet Reg Health Eur. 2023 Oct 26;34:100737. doi: 10.1016/j.lanepe.2023.100737. PMID: 37927440; PMCID: PMC10625009.
- [23]. Shu Y, Zhang Q, He X, Liu Y, Wu P, Chen L. Fluoroquinolone-associated suspected tendonitis and tendon rupture: A pharmacovigilance analysis from 2016 to 2021 based on the FAERS database. Front Pharmacol. 2022 Sep 6;13:990241. doi: 10.3389/fphar.2022.990241. PMID: 36147351; PMCID: PMC9486157.
- [24]. Gorelik E, Masarwa R, Perlman A, Rotshild V, Abbasi M, Muszkat M, Matok I. Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-analysis and Network Meta-analysis. Drug Saf. 2019 Apr;42(4):529-538. doi: 10.1007/s40264-018-0751-2. PMID: 30368737.
- [25]. Abo-Salem E, Fowler JC, Attari M, et al. Antibiotic-induced cardiac arrhythmias. Cardiovasc Ther. 2014;32(1):19-25. doi:10.1111/1755-5922.12054
- [26]. Doyle M, Vodstrcil LA, Plummer EL, et al. Nonquinolone Options for the Treatment of Mycoplasma genitalium in the Era of Increased Resistance. Open Forum Infect Dis. 2020;7(8):ofaa291. doi:10.1093/ofid/ofaa291.
- [27]. Horner P, Donders G, Cusini M, et al. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women? – a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol. 2018;32(11):1845-1851.
- [28]. Centers for Disease Control and Prevention. Urethritis & Cervicitis – 2015 STD Treatment Guidelines [Internet]. Atlanta (GA): CDC; [cited 2024 Mar 14]. Available from: https://www.cdc.gov/std/treatment-guidelines/urethritis-and-cervicitis.htm
- [29]. Rumyantseva, T., Khayrullina, G., Guschin, A., & Donders, G. (2019). Prevalence of Ureaplasma spp. and Mycoplasma hominis in healthy women and patients with flora alterations. Diagnostic Microbiology And Infectious Disease. 93(3), 227-231
- [30]. Fernández J, Karau MJ, Cunningham SA, Greenwood-Quaintance KE, Patel R. Antimicrobial Susceptibility and Clonality of Clinical Ureaplasma Isolates in the United States. Antimicrob Agents Chemother. 2016;60(8):4793-4798. doi:10.1128/AAC.00671-16.
- [31]. Beeton, M. L., Payne, M. S., & Jones, L. (2019). The role of Ureaplasma spp. in the development of nongonococcal urethritis and infertility among men. Clinical microbiology reviews, 32(4), 10-1128.
- [32]. Ahmed, J., Rawre, J., Dhawan, N., Khanna, N., Dhawan, B. Mycoplasma hominis: An under recognized pathogen. Indian Journal of Medical Microbiology. 2021; 39(1), 88-97.
- [33]. Taylor-Robinson D. Mollicutes in vaginal microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Research in Microbiology. (2017).168(9-10), 875–881. https://doi.org/10.1016/j.resmic.2017.02.009
- [34]. Smolec D, Ekiel A, Kłuciński P, Kawecki J. Occurrence of urogenital mycoplasmas in men with the common genitourinary diseases. Braz J Microbiol. 2021;52(4):2013-2019. doi:10.1007/s42770-021-00620-1
- [35]. Rechnitzer H, Brzuszkiewicz E, Strittmatter A, et al. Genomic features and insights into the biology of Mycoplasma fermentans. Microbiology. 2011;157(Pt 3):760-773. doi:10.1099/mic.0.043208-0
- [36]. Sim KY, Byeon Y, Bae SE, Yang T, Lee CR, Park SG. Mycoplasma fermentans infection induces human necrotic neuronal cell death via IFITM3-mediated amyloid-β (1-42) deposition. Sci Rep. 2023;13(1):6864. Published 2023 Apr 26. doi:10.1038/s41598-023-34105-y
- 37. Waites KB, Crabb DM, Ratliff AE, et al. Latest Advances in Laboratory Detection of Mycoplasma genitalium. J Clin Microbiol. 2023;61(3):e0079021. doi:10.1128/jcm.00790-21.
- 38. Centers for Disease Control and Prevention. Pelvic Inflammatory Disease (PID) – STI Treatment Guidelines. Published September 21, 2021. Accessed from www.cdc.gov.
Down the article PDF: A Review of Genital Mycoplasma and Ureaplasma Infections for the Urgent Care Clinician
