Batsheva R. Sholomson, DO; Danielle Langan, DO; Abbas Husain, MD; Shorok Hassan, DO
Urgent Message: With the incidence of babesiosis rising, clinicians are encouraged to consider the totality of presentation including risk factors based on endemic region, recent travel or tick bite, and clinical signs and symptoms.
Citation: Sholomson BR, Langan D, Husain A, Hassan S. Fever of Unknown Origin: A Case Report of Babesiosis Infection. J Urgent Care Med. 2024; 18(10):17-21
Key Words: Babesiosis, parasites, ticks, hemolytic anemia, hemolysis, fevers
Abstract
Introduction: Babesiosis is a tick-borne, zoonotic parasitic infection of the red blood cells that can present with a variety of non-specific signs and symptoms.
Clinical presentation: A 65-year-old man with a past medical history of diabetes and atrial fibrillation presented to an emergency department with a persistent fever for 10 days. The patient noted that he hiked frequently in the woods. On presentation, his vital signs were notable for a temperature of 102.6°F (39.2°C) and a heart rate of 120 beats per minute.
Work-Up and Diagnosis: On laboratory assessment, abnormalities identified by the treating clinicians includedanemia, thrombocytopenia, and elevated liver enzymes. The pattern fit that of a hemolytic anemia. A Babesia polymerase chain reaction test was positive, and the peripheral blood smear showed intracellular red blood cell parasites consistent with Babesia infection.
Resolution: The patient was treated for a total of 10 days with oral atovaquone 750 mg twice daily and azithromycin 500 mg once daily. His parasitemia on peripheral blood smear subsided on repeat testing.
Conclusion: The incidence of babesiosis has been rising with the potential to manifest as a life-threatening illness. This case highlights the importance of considering the totality of presentation including risk factors based on endemic region, recent travel or tick bite, and clinical signs and symptoms.
Introduction
Babesiosis is a zoonotic disease caused by many different Babesia species that infect a wide array of wild and domestic animals serving as vertebrate reservoirs. Humans are incidental hosts wherein only a few Babesia species are known to cause diseases in humans. Specifically, Babesia microti is the species most commonly found to affect people in the United States.1 Babesiosis is a parasitic infection of the red blood cells, and the incidence of babesiosis has been rising with the potential to manifest as a life-threatening illness.2 Being able to identify signs and symptoms in the urgent care (UC) or emergency department (ED) setting is crucial to making the diagnosis and starting appropriate and timely treatment. This article will focus on the key areas of recognition, evaluation, testing, management, and, ultimately, treatment of babesiosis.
Case Presentation
A 65-year-old man with a past medical history of diabetes mellitus and atrial fibrillation presented to an ED with a persistent fever for 10 days. The maximum recorded temperature at home was 103°F (39.4°C). Associated symptoms included chills, fatigue, nausea, and weight loss of 8lbs. The patient noted that he frequently hiked in the woods. He denied chest pain, cough, difficulty breathing, vomiting, diarrhea, abdominal pain, dysuria, recent travel, known tick bites, or sick contacts.
Physical Exam
On presentation, his vital signs were notable for a temperature of 102.6°F (39.2°C), heart rate of 120 beats per minute, blood pressure of 157/79 mmHg. His respiratory rate and oxygen saturation were normal. He was non-toxic appearing and in no distress. He had no conjunctival injection, rhinorrhea, pharyngeal erythema, or abnormalities of tympanic membranes. On cardiovascular exam, he had an irregular rate and rhythm with no murmurs. His abdominal exam and pulmonary exam were normal. He had no rash. On neurological assessment, he was alert and answered questions appropriately, and had no focal deficits.
The patient had extensive testing for undifferentiated fever of unknown origin including complete blood count (CBC) with differential, complete metabolic panel, blood cultures, urinalysis and urine culture, chest x-ray (CXR), inflammatory markers, lactate dehydrogenase (LDH), haptoglobin, and peripheral blood smears.
His laboratory results were significant for: 1) normocytic anemia with hemoglobin level of 13.6 g/dL, hematocrit of 39.5%, MCV of 87.8 fL; 2) thrombocytopenia with platelet count of 74 x 109/L; 3) a hemolysis patter with elevated LDH of 602 U/L and haptoglobin of <20 mg/dL and mild hyperbilirubinemia with total bilirubin of 1.5 mg/dL, indirect bilirubin of 1.2 mg/dL, and direct bilirubin of 0.3 mg/dL; 4) mildly elevated liver enzymes with AST of 43 U/L and ALT of 42 U/L; 5) lymphopenia with atypical lymphocytes and reactive lymphocytes. The CXR was unremarkable, and a Babesia polymerase chain reaction (PCR) was positive. The peripheral blood smear showed parasitemia of 4.9%. Blood culture and urine cultures showed no growth.
Medical Decision Making
Without a focal source, a broad laboratory evaluation is indicated in the setting of prolonged fever. Commonly available laboratory tests can aid in the diagnosis of babesiosis. Characteristic abnormalities include anemia, thrombocytopenia, and elevated liver enzymes. Patients can often present with a pattern of hemolysis (ie, elevated LDH, low haptoglobin, elevated bilirubin).1
Differential Diagnosis and Final Diagnosis
A differential diagnosis for the presentation of persistent fever of unknown origin for at least 10 days (as was the case with this patient) includes meningitis, encephalitis, bacterial or fungal pneumonia, intra-abdominal infection, urinary tract infection, skin or soft tissue infection, malaria, venous thromboembolism, autoimmune or oncologic processes, bacteremia, and other tick-borne illnesses (eg, Lyme disease, ehrlichiosis).3
The social history of hiking frequently and laboratory results demonstrating hemolytic anemia raised a strong suspicion for tick-borne illnesses, including babesiosis.
The presence of a single positive Babesia-specific antibody test is not sufficient to establish a diagnosis. Babesia antibodies can persist in the blood for years despite resolution of an infection, with or without treatment. Confirmatory testing includes peripheral blood smear showing intraerythrocytic rings and/or Maltese cross which represents Babesia parasites or positive detection of Babesia DNA on PCR.1
The patient had both a positive Babesia PCR and a peripheral blood smear that showed intracellular red blood cell parasites consistent with Babesia infection.
In cases of suspected babesiosis, testing for Lyme titers should also be performed. Lyme disease is also caused by ticks, and there is a likelihood of coinfection.1 The patient had negative testing for Lyme PCR.
Discussion
Babesiosis is caused by microscopic parasites that infect red blood cells and are most commonly spread by the bite of an infected Ixodes scapularis, also known as the black-legged tick or deer tick. Less common forms of transmission include blood transfusion, organ transplantation, or congenitally.2 Babesiosis occurs via tick borne transmission and most commonly is associated with exposure to overgrown grassy fields and leaf piles. Protection against contracting babesiosis includes wearing long pants and long-sleeved shirts, applying tick repellants to skin and clothing, and performing tick checks after possible exposure.5
Tick borne illnesses are most common in the Northeast and upper Midwest, with peak season during the warm months. Overall, U.S. tick borne disease cases have increased 25%, from 40,795 reported in 2011 to 50,856 in 2019. During 2011–2019, a total of 16,456 cases of babesiosis were reported to the Centers for Disease Control and Prevention (CDC) by 37 states. The CDC previously considered babesiosis to be endemic in the following 7 states: Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Minnesota, and Wisconsin. There has additionally been a large increase in incidence of babesiosis between 2011 and 2019 in Maine, New Hampshire, and Vermont, and the CDC now also considers babesiosis to be endemic in these states as well.4 In 2020, the reportable cases of babesiosis to the CDC decreased by 24% compared to 2019. The impact of the COVID-19 pandemic could influence actual case numbers more than is reported.5 The actual number of cases is difficult to quantify as it is thought that there is an underestimation due to asymptomatic infection, failure to report cases, and misdiagnosis.6
Babesiosis has been reported in North and South America, Europe, Asia, Africa, and Australia. Some of the countries affected are susceptible to different endemic species whereas others merely have sporadic cases. The predominant species to affect humans, Babesia microti, is endemic to the United States.7
Babesiosis has a range of presentation from asymptomatic to severe. Babesiosis will typically occur after an incubation period of 1 to 4 weeks after inoculation and can last several weeks.2 Signs and symptoms include: fever, chills, sore throat, nonproductive cough, dyspnea, nausea, vomiting, abdominal pain, headache, myalgia, arthralgia, neck stiffness, emotional liability and depression, hyperesthesia, conjunctival injection, photophobia, fatigue, sweats, loss of appetite, and weight loss.6
Babesia protozoa invade and cause lysis of red blood cells (RBC) resulting in hemolytic anemia, which can present with jaundice, dark urine, and pallor.5 The severity of symptoms often correlates with the parasitemia level. Parasitemia <4% will often present mild to moderate, and parasitemia ≥4% will often present with severe illness. Complications include acute respiratory distress syndrome, congestive heart failure, splenomegaly, hepatomegaly, renal failure, liver failure, disseminated intravascular coagulation, severe anemia, or even rarely death.1 These complications can be devastating in immunocompromised populations including asplenia, HIV/AIDS, malignancy, immunosuppressive therapy, neonates, and elderly patients.2
The early diagnosis of symptomatic patients helps prevent delays in treatment and potential complications.
Babesiosis can be included in the differential of any patient who presents with typical signs and symptoms, expected laboratory abnormalities, and lives in or has traveled to a Babesia endemic region within the past month or received a blood transfusion within the past 6 months. If your suspicion is low and the individual has not recently traveled to an endemic area, further pursuit of testing for babesiosis is not warranted.1 If there is clinical suspicion in primary care or urgent care settings where testing cannot be performed, then the patient should be referred to a facility with greater testing capabilities. In clinically stable patients without other suspicions for serious illness (eg, sepsis etc.), ED referral may be foregone if laboratory results can be expected within 24-48 hours. This approach is best reserved for lower risk patients and if discharging patients while laboratory results are pending, however, strict ED precautions for worsening symptoms are prudent to review.
In patients who are immunocompetent and asymptomatic for babesiosis, it is reasonable to defer treatment and closely monitor the patient. Treatment is indicated for asymptomatic patients who have a positive blood smear with parasitemia for more than a month.1 If patients are mildly to moderately ill or are at a higher risk for severe or relapsing infection, providers should start medications in ambulatory care. If patients are severely ill, providers should refer the individual to an ED for further evaluation and treatment of the infection as well as possible secondary complications.1 Treatment should ultimately be tailored to each individual patient.
Additional studies are needed to assess whether suggestive laboratory abnormities on CBC, liver enzymes, and markers of hemolysis are sufficiently sensitive to use for screening purposes prior to the decision to order babesiosis specific laboratory testing.1 Confirmatory testing specific for babesiosis includes peripheral blood smear or PCR. It is important to request a manual review of the blood smear (nonautomated) and recognize that multiple smears may need to be examined.5
Further studies are also needed to determine whether peripheral blood smear or PCR serves as the better initial test used to diagnose babesiosis.Blood smear examination is rapid and inexpensive. In the event of low parasite burden or negative testing on blood smears, however, PCR testing is helpful.1 Real-time PCR has been shown to be more sensitive and specific compared to the microscopic examination of blood smears.8
Many tick-borne illnesses are treated with doxycycline including: Lyme disease, Rocky Mountain spotted fever, anaplasmosis, ehrlichiosis, and southern tick-associated rash illness.9 Unfortunately, doxycycline is not recommended for babesiosis. Hence, it is important to consider babesiosis and specific testing for this in the setting of fever and suspected tick-related illness as babesiosis may not adequately be treated if doxycycline is given empirically.1
Per the Infectious Diseases Society of America, the treatment for babesiosis includes varying combinations of an antiparasitic agent and an antibiotic agent. The preferred, first-line treatment is a combination of atovaquone and azithromycin for mild to moderate illness. The alternative treatment for severe illness is a combination of quinine and clindamycin. Treatment is typically for 7 to 10 days, but may be extended for immunocompromised populations (Table 1).1
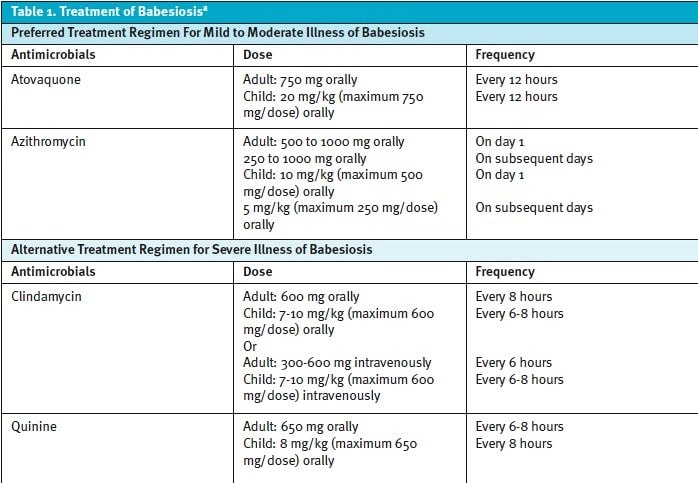
If the patient is severely ill, other treatment options that may be indicated include blood transfusion, exchange transfusion, mechanical ventilation, and/or dialysis depending on the end-organ dysfunction and degree of anemia present. Exchange transfusion of blood cells is recommended for patients with parasitemia ≥10% or severe hemolytic anemia with a hemoglobin of <10 g/dL, or severe pulmonary, renal, or hepatic compromise. Exchange transfusion can rapidly decrease parasite levels in the blood, corrects anemia, and removes toxic byproducts of Babesia.10
Disposition
The patient was ultimately admitted to the hospital. The infectious disease specialist was consulted. The patient received 10 days of atovaquone 750 mg twice daily and azithromycin 500 mg once daily. Parasitemia on peripheral blood smear subsided with repeat testing 2 days after admission (2.6%) and further still by 5 days after admission (1.9%). The patient quickly defervesced, and the laboratory markers and clinical symptoms improved significantly. He was discharged home without complication.
Ethics Statement
Patient was unable to be contacted because he was lost to follow-up. Some patient demographics were changed to protect patient privacy and confidentiality.
Takeaway Points
- Human babesiosis is a tick-borne zoonotic illness caused by parasites transmitted primarily by ticks in grassy and wooded areas; the incidence and geographic distribution of the disease has been increasing.
- Babesia infections can range in severity from asymptomatic to fatal; immunocompromised and elderly populations are at the highest risk of complications.
- Prolonged fever of unknown origin and other symptoms may mimic many other infectious and non-infectious etiologies.
- Characteristic laboratory abnormalities include anemia, thrombocythemia, elevated liver enzymes, and markers of hemolysis.
- Totality of presentation with risk factors based on living or travel to endemic regions, risk of tick bite, and clinical signs and symptoms can raise concern for babesiosis.
- In the urgent care, immediate laboratory tests may not be available. If babesiosis is suspected, treat the patient appropriately by ordering appropriate screening and confirmatory laboratory testing with either peripheral blood smear or positive detection of Babesia DNA on PCR in stable patients. More seriously ill appearing and higher risk patients should be referred immediately to an ED.
Manuscript submitted March 19, 2024; accepted June 7, 2024.
References
- Krause PJ, Auwaerter PG, Bannuru RR, Branda JA, Falck-Ytter YT, Lantos PM, Lavergne V, Meissner HC, Osani MC, Rips JG, Sood SK, Vannier E, Vaysbrot EE, Wormser GP. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA): 2020 Guideline on Diagnosis and Management of Babesiosis. Clin Infect Dis. 2021 Jan 27;72(2):185-189. doi: 10.1093/cid/ciab050.
- Waked R, Krause PJ. Human Babesiosis. Infect Dis Clin North Am. 2022 Sep;36(3):655-670. doi: 10.1016/j.idc.2022.02.009.
- Roth AR, Basello GM. Approach to the adult patient with fever of unknown origin. Am Fam Physician. 2003;68(11):2223-2228.
- Swanson M, Pickrel A, Williamson J, Montgomery S. Trends in Reported Babesiosis Cases—United States, 2011–2019. MMWR Morb Mortal Wkly Rep 2023;72:273–277. DOI: http://dx.doi.org/10.15585/mmwr.mm7211a1.
- Centers for Disease Control and Prevention. Parasites – Babesiosis. Accessed on June 3, 2023. https://www.cdc.gov/parasites/babesiosis/.
- Krause PJ. Human babesiosis. Int J Parasitol. 2019;49(2):165-174. doi:10.1016/j.ijpara.2018.11.007.
- Kumar A, O’Bryan J, Krause PJ. The Global Emergence of Human Babesiosis [published correction appears in Pathogens. 2022 May 23;11(5):] [published correction appears in Pathogens. 2022 Aug 17;11(8):]. Pathogens. 2021;10(11):1447. Published 2021 Nov 6. doi:10.3390/pathogens10111447.
- Wang G, Wormser GP, Zhuge J, et al. Utilization of a real-time PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick Borne Dis. 2015;6(3):376-382. doi:10.1016/j.ttbdis.2015.03.001.
- Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med (Lond). 2015 Feb;15(1):74-7. doi: 10.7861/clinmedicine.14-6-74.
- Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015 Jun;29(2):357-70. doi: 10.1016/j.idc.2015.02.008.
Author Affiliations: Batsheva R. Sholomson, DO, Staten Island University Hospital in Staten Island, New York. Danielle Langan, DO, Staten Island University Hospital in Staten Island, New York. Abbas Husain, MD, Staten Island University Hospital in Staten Island, New York. Shorok Hassan, DO, Staten Island University Hospital in Staten Island, New York. Authors have no relevant financial relationships with any ineligible companies.
Download the article PDF: Fever of Unknown Origin: A Case Report of Babesiosis Infection
