Urgent message: When a relatively young patient presents to urgent care with chest pain, there may or may not be a “typical” cause. Prompt evaluation and accurate assessment of risk factors are essential to efficient care and, often, the patient’s survival.
Max Palatnik, MD
Case Presentation
A 35-year-old male presented at 21:59 with a chief complaint of chest pain; at 22:03, we noted the following:
Temp: 98.9
Pulse: 103
Resp: 16
Syst: 122
Diast: 69
O2Sat: 99%
History of Present Illness (22:47) (Verbatim)
Pt. 38 year old male with a PMH of myocarditis and pericarditis in 1983 and 1991, who ate dinner at 6:30 and began feeling pressure across his anterior chest while watching TV at 7:30 PM. – it felt like “some was sitting on my chest”. Associated SOB and radiation into his shoulder and left hand “tingling”. He has had heartburn but this felt different. Took baking soda (which he normally takes for his heartburn) and this did not help. No syncope, nausea, vomiting, fever, RUQ pain or history of food intolerances. He did have some viral symptoms 2 weeks ago (nonproductive cough, sinus HA and PND which has all resolved.) No orthopnea, PND, relation of pain to exercise, chest trauma, pleuritic component
Past Medical History/Triage
Medication, common allergies: None
PMH: Myocarditis/Pericarditis
PSH: None
SocHx: Non-smoker
FamHx: Positive for CAD with 52 year old sibling with MI, father CABG at 53
Exam (22:52)
General: Well-appearing; well-nourished; in no apparent distress.
Head: Normocephalic; atraumatic.
Eyes: PERLA; EOM intact
ENT: TM’s normal; normal nose; no rhinorrhea; Throat is red, and mild exudates.. Moist mucus membranes.
Neck: Supple; nontender; no cervical lymphadenopathy. No meningeal signs
Cardiovascular: Normal S1, S2; no murmurs, rubs, or gallops. No reproducible chest wall tenderness
Respiratory: Normal chest excursion with respiration; breath sounds clear and equal bilaterally; no wheezes, rhonchi, or rales.
Abdomen: Normal bowel sounds; non-distended; nontender; no palpable organomegaly.
Extremities: Normal ROM in all four extremities; nontender to palpation; distal pulses are normal and equal.
Skin: Normal for age and race; warm; dry; good turgor; no apparent lesions or exudate
Progress Notes (23:12)
He received 2 baby aspirin and SL NTG with relief of chest discomfort. He then had 1 inch of Nitropaste placed. At 00:44 his pain returned and his ECG was repeated. He was given 15mg Maalox without improvement then ½ inch more NTP which did relieve the discomfort.
Results
EKG 1: Flattened T waves inferior and in V2-V6.
EKG 2: No changes
Labs
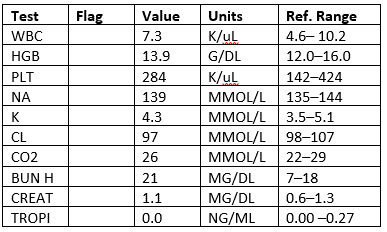
CXR: Negative
Diagnosis (01:57)
Chest pain, history of myocarditis
Disposition (02:02)
The patient was admitted to the hospital under telemetry.
Hospital Course
Pt. underwent serial enzymes and repeat ECG in the morning. He ruled out for MI and was released. A subsequent stress ECHO was negative after exercising for 12.5 minutes with no chest discomfort or ECG changes.
Second Visit
- The patient followed up with his PCP, was diagnosed with GERD, and was started on a PPI.
- The patient returned to the ED 6 weeks later with chest tightness and dyspnea in the setting of a meal. His symptoms were worsened by exertion, occur at rest, and are improved by upright positioning as well as with Prilosec.
- No associated fever, cough, radiation, diaphoresis, calf pain, peripheral edema.
- The patient has normal vital signs, with an unremarkable, appropriate examination.
- EKG reveals TWI in aVL, as well as new q waves in V1-V2 and NSST changes.
- CXR is negative.
- Labs show a troponin that is >20 times the upper limit of normal.
- The patient receives aspirin, heparin, Plavix, nitroglycerin and is admitted for acute coronary syndrome.
- The patient undergoes percutaneous coronary intervention (PCI) with successful stent placement and is subsequently discharged in good condition.
Discussion
Chest pain is the presenting complaint for more than 5% of the patients in emergency departments (EDs) in the United States. The evaluation of the patient with chest pain is a tremendous challenge, largely due to the broad differential diagnosis, but also because of the risk associated with misdiagnosis. Among the most rapidly fatal conditions in emergency medicine—many of which may present initially to urgent care—are acute coronary syndrome (myocardial ischemia and infarction), aortic dissection, pulmonary embolism, pericarditis with cardiac tamponade, myocarditis, tension pneumothorax, and esophageal rupture. All of these conditions tend to manifest with chest pain, and they all should be considered early in the evaluation of the patient with chest pain.
It is the responsibility of the physician to evaluate these patients with the list of potential life-threats at the top of the differential diagnosis. A detailed history, physical examination (with focus on cardiac, pulmonary, and vascular examinations), and basic testing information (eg, electrocardiogram, chest radiograph) can often rapidly rule out these life threats with reasonable accuracy. However, in cases where this initial rapid assessment fails to rule out one of the deadly diagnoses, further workup in a higher-acuity setting may be warranted.
Although typical symptoms of ACS are described as a gradual onset of aching or pressure pain in the chest with radiation to the left arm, neck or jaw, in truth the atypical may be more “typical.” In a large data synthesis, the most helpful historical features that increased the likelihood of acute myocardial infarction were radiation to the right arm or shoulder, radiation to both arms, pain that worsened with exertion, diaphoresis, and nausea or vomiting.1 Certain subsets, including women, diabetics, and elderly are more likely to have anginal symptoms that are represented by dyspnea, vomiting, diaphoresis, generalized weakness; some may have painless presentations.2 Even in those patients with atypical symptoms such as pleuritic pain or palpable tenderness in the chest wall, the posttest likelihood is only sufficiently lowered in those who are already low risk.1 Enumeration of historical cardiac risk factor burden is of little prognostic value, especially in patients older than 40 years old.3
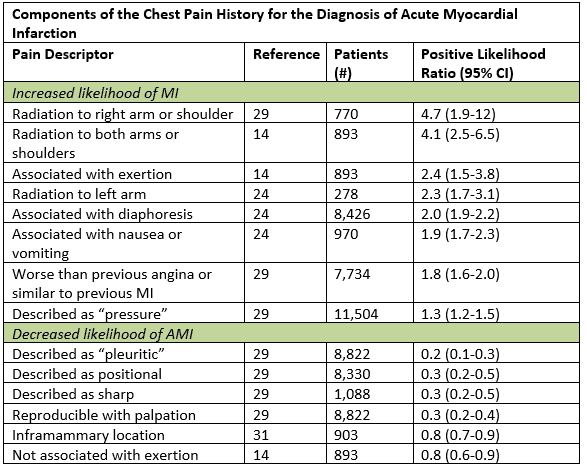
Adapted from Swap CJ, et al. JAMA. 2005;294:2623-2629.
Young patients (<45 years-old) represent a group at high risk for misdiagnosis of ACS, primarily because of a tendency on the part of physicians to underestimate cardiac risk. Up to 10% of myocardial infarctions in the U.S. occur in patients <45 years of age, the majority of which are related to atherosclerotic heart disease. Atherosclerotic disease was noted in 17% of teenagers in one study,4 and multivessel disease noted in 20% of young adults (average age: 26 years) in an autopsy study of victims of inner city violence.5 A recent ED study found that 5.4% of patients 24─39-years-old presenting with chest pain ruled in for ACS, and 2.2% had an adverse cardiac event (ie, death, MI, need for percutaneous coronary intervention or cardiac bypass surgery) within 30 days.6 Although the overall incidence of ACS is lower in young patients, physicians should not discount a concerning HPI based purely on a patient’s age.
Diabetes mellitus (DM) represents another high-risk condition in terms of potential for misdiagnosis of ACS. Patients with DM are prone to painless presentations when they have cardiac ischemia. Atypical presentations (eg, dyspnea, confusion, emesis, fatigue) occur in up to 40% of cases. Diabetic patients are also more likely to have adverse outcomes from ACS.7 Treating physicians must therefore not rely on typical presenting complaints to initiate a cardiac workup in diabetic patients, nor should they rely on positive cardiac biomarkers to prompt an aggressive approach to treatment in these patients.
Cocaine use must be considered an additional independent risk factor for atherosclerotic heart disease and MI, especially in young patients. Some authors estimate that cocaine accounts for up to 25% of acute MIs in patients <45 years.7 Acute use of cocaine can induce coronary vasoconstriction, increased platelet aggregation, and/or adrenergic stimulation leading to dysrhythmias and ischemia. Chronic use of cocaine is associated with MI, as well, causing markedly accelerated atherogenesis and subsequent early MI. Overall, cocaine users have a seven-fold increased risk of MI.8
Systemic lupus erythematosus (SLE) is a significant but underappreciated risk factor for early atherosclerosis and myocardial infarction. Young patients with SLE are estimated to have a nine-fold increased risk of early MI.9 Women <45 years, in particular, are at increased risk, with estimates of increased risk of early MI as high as fifty-fold.10 The cause of premature atherosclerosis in SLE is likely multifactorial, but largely related to coexisting systemic inflammation and dyslipidemias.
Human immunodeficiency virus (HIV) infection has been identified as an independent risk factor for premature atherosclerosis, as well. Evidence suggests that HIV infection causes endothelial injury to coronary vessels, initiating an inflammatory cascade leading to atherosclerotic lesions.11 The finding of premature atherosclerosis is especially prominent in patients with later stages of HIV infection (CD4 count <200).12 The medication regimens that are currently used in treating HIV (protease inhibitors) also exacerbate the risk of early atherosclerosis. Overall, HIV patients with ACS present at an age that is more than 10 years younger than non-HIV patients.13
Chronic renal disease (CRD) has also recently been identified as an independent risk factor for accelerated atherosclerosis. CRD is associated with chronic inflammation14 and increased platelet aggregation.15 These factors, combined with an increased prevalence of concomitant conventional risk factors, produce a disproportionately high risk of cardiac events in these patients.14
Though it should be obtained and evaluated within 10 minutes of presentation concerning for cardiac ischemia, the ECG should not be used to rule out ACS. Up to 50% of patients with cardiac ischemia or infarction will have a nonspecific or normal ECG.16 Serial ECGs can increase the diagnostic yield at confirming the presence of ACS in patients with ongoing symptoms.
Much like the ECG, cardiac biomarkers are useful when they are positive, but have limited utility when they are normal. Serial biomarker testing over the course of 3─6 hours has become a routine protocol in many EDs and has excellent sensitivity at detecting evidence of MI. However, biomarkers cannot be relied upon to rule out cardiac ischemia. Prospective validation of the “HEART” score has afforded practitioners the ability to reliably differentiate a subset of low-risk patients that would likely not benefit from additional testing.17
Stress testing and coronary angiography are being used more commonly early in the evaluation of patients with chest pain to rule in ACS. Although a negative stress test or angiogram is associated with a lower risk of underlying CAD, neither test can definitively rule out ACS or the presence of significant underlying coronary thromboses. The majority of stress testing modalities detect evidence of significant coronary lesions with only 85% to 95% sensitivities.18-19 Coronary angiography is also an imperfect test; false negative angiography interpretations are not uncommon in the presence of diffuse disease, eccentric plaques, “flush” occlusions, branch ostial lesions, overlapping side branches, and even when lesions are present within the left main coronary artery.20 Further compromising the reliability of these tests are data indicating that the majority of MIs occur from occlusions within arteries that were previously <50% obstructed before the infarct occured.21-23
These types of lesions are usually associated with negative stress tests or “nonsignificant” angiograms if the tests are done prior to infarct. By their nature of detecting fixed coronary stenosis, stress tests are unable to evaluate for or predict vulnerable coronary plaques which are at risk for becoming dislodged, leading to an acute coronary event. In one study of patients being evaluated for ACS who had a negative stress test in the prior 3 years, over 20% reached the composite index of AMI, positive stress test, CABG or catheterization with intervention, with the vast majority of negative testing occurring within 1 year of presentation.24 Similarly, on even more invasive testing men and women with either normal or “minimal” CAD on heart catheterization (1.2% and 3.3%, respectively) had either AMI or death within 1 year of follow-up.25 It is vital that the clinician does not rely on prior objective testing in the face of a patient with signs and symptoms indicative of ACS.
Summary
The evaluation of chest pain and possible ACS is a high-risk endeavor. The decision to pursue a “full cardiac workup” should primarily be based on a thorough HPI. Physicians should be aware of the frequency of atypical presentations, especially in women, elderly, and diabetic patients. Young patients also deserve special consideration, as their risk is often underappreciated. Additional nontraditional cardiac risk factors, including cocaine, systemic lupus erythematosus, human immunodeficiency virus, and chronic renal disease warrant extra attention. Diagnostic testing consists of electrocardiography (helpful to rule in ACS, but not to rule out the diagnosis) and cardiac biomarker testing, which are also primarily useful when positive. Importantly, the negative stress test or angiogram is very helpful at stratifying patients to a low risk of ACS and CAD, but they do not definitively rule out the diagnosis.
Citation: Palatnik M. A 38-year-old man with chest pain. J Urgent Care Med. March 2018. Available at: https://www.jucm.com/a-38-year-old-man-with-chest-pain/.
References
- Swap CJ, Nagurney JT. Value and Limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294:2623-2629.
- El-Menyar A, Zubaid M, Sulaiman K. Atypical presentation of acute coronary syndrome: A significant independent predictor of in-hospital mortality. J Cardiol. 2011;57;165-171.
- Han JH, Lindsell CJ, Storrow AB, et al. The role of cardiac risk factor burden in diagnosing acute coronary syndromes in the emergency department setting. Ann Emerg Med. 2007;49;145-152.
- Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103:2705-2710.
- Joseph A, Ackerman D, Talley JD, et al. Manifestations of coronary atherosclerosis in young trauma victims—an autopsy study. J Am Coll Cardiol. 1993;222:459-467.
- Marsan RJ Jr., Shaver KJ, Sease KL, et al. Evaluation of a clinical decision rule for young adult patients with chest pain. Acad Emerg Med. 2005;12:26-32.
- Fergus TS, Fazel R, Fang J, et al. Presentation, management, and outcomes of diabetic patients compared to non-diabetic patients admitted for acute coronary syndromes. Heart. 2004;90:1051-1052.
- Qureshi AI, Suri MF, Guterman LR, et al. Cocaine use and the likelihood of nonfatal myocardial infarction and stroke: data from the Third National Health and Nutrition Examination Survey. Circulation. 2001;103:502-506.
- D’Agate DJ, Kokolis S, Belilos E, et al. Premature coronary artery disease in systemic lupus erythematosus with extensive reocclusion following coronary artery bypass surgery. J Invasive Cardiol. 2003;15:157-163.
- Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408-415.
- Varriale P, Saravi G, Hernandez E, et al. Acute myocardial infarction in patients infected with human immunodeficiency virus. Am Heart J. 2004;147:55-59.
- Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603-1608.
- Hsue PY, Giri K, Erickson S, et al. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109:316-319.
- Yerkey MW, Kernis SJ, Franklin BA, et al. Renal dysfunction and acceleration of coronary disease. Heart. 2004;90:961-966.
- Aggarwal A, Kabbani SS, Rimmer JM, et al. Biphasic effects of hemodialysis on platelet reactivity in patients with end-stage renal disease: a potential contributor to cardiovascular risk. Am J Kidney Dis. 2002;40:315-322.
- Brady WJ, Aufderheide TP, Chan T, et al. Electrocardiographic diagnosis of acute myocardial infarction. Emerg Med Clin North Am. 2001;19:295-320.
- Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiology. 2013;168;2153-2158.
- Ioannidis JPA, Salem D, Chew PW, et al. Accuracy of imaging technologies in the diagnosis of acute cardiac ischemia in the emergency department: a meta-analysis. Ann Emerg Med. 2001;37:471-477.
- Lateef F, Gibler WB. Provocative testing for chest pain. Am J Emerg Med. 2000;18:793-801.
- Schwartz L, Gourassa MG. Evaluation of patients with chest pain and normal coronary angiograms. Arch Int Med. 2001;161:1825-1833.
- Giroud D, Li JM, Urban P, et al. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992;69:729-732.
- Hackett D, Davies G, Maseri A. Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur Heart J. 1988;9:1317-23.
- Hackett D, Verwilghen J, Davies G, et al. Coronary stenoses before and after acute myocardial infarction. Am J Cardiol. 1989;63:1517-1518.
- Walker J, Galuska M, Vega D. Coronary disease in emergency department chest pain patients with recent negative stress testing. West J Emerg Med. 2010;11;384-388.
- Hemingway H, McCallum A, Shipley M, et al. JAMA 2006;295;1404-1411.
