Urgent message: Patients and providers alike may be inclined to eliminate possible diagnoses based on first impressions. It is essential that the urgent care clinician maintain a wide differential when evaluating patients for new-onset complaints that do not have an obvious cause. Failure to do so may cause delayed diagnoses (and, consequently, delayed treatment).
Haley Harrington, Ross L. Pearlman, MD and Robert T. Brodell, MD
INTRODUCTION
Ticks are small—so small that patients often do not feel their bite or sense their presence once it is attached. As such, attached ticks can be mistaken by patients as “new moles.”1,2 The diseases they carry, including Lyme disease, Rocky Mountain spotted fever, tularemia, and ehrlichiosis, cause significant morbidity and mortality.3 It is important that physicians recognize and remove ticks promptly to decrease the potential for disease transmission.4,5 Here, we present a case of a tick mistaken for a growing pigmented lesion.
CASE PRESENTATION
A 46-year-old female presented for a new mole “she just noted” on her back. She reports that the mole had been “growing” over the past few days. There was no itching, burning, or bleeding. On review of systems, she denied constitutional symptoms and arthralgia or myalgia. The patient had no history of recent outdoor exposures or any overseas travel. Her past medical history was significant for hypertension, managed with diet and exercise. No medications. She reported a family history significant for malignant melanoma in her father. The patient denied smoking and reported drinking socially on weekends.
Physical examination revealed a 2 mm brown-black papule on the lower back (Figure 1) without surrounding erythema. No other cutaneous abnormalities were found on full skin exam. Examination of cervical, axillary, and inguinal lymph nodes was unremarkable. Close macroscopic inspection of the papule revealed thin, spindle-like projections adjacent to the black-brown papule, and a tick was identified.
OUTCOME
The insect was grasped with sterile forceps adjacent to the skin at the level of the mouth parts and gently lifted away from the skin and extracted en bloc. This tick was found to be an American Dog tick, Dermacentor variabilis, which is a member of the Ixodidae family.
DISCUSSION Patients may mistake a tick for a new skin lesion; as ticks are often hidden by hair or skin folds, other patients may be completely unaware of tick attachment. Ticks inject a variety of chemicals to prevent an inflammatory response, facilitate the blood meal, and allow for a painless bite and disease transmission. In order for a tick to successfully transmit infection to a human, it must first feed on an animal that is infected, as ticks are the intermediate host in most cases. After feeding, the bacteria are ingested and travel to the gut of the tick for maturation. Once maturation of the bacteria is complete, systemic infection may occur. The bacteria infect the salivary glands and from there can be transmitted to the next host via a subsequent tick bite.6 Risk of infection increases with tick attachment over 72 hours, but transmission can occur in the first 16 hours.7,8 If a patient presents with a tick, it is important to completely remove the tick to prevent disease transmission.9,10
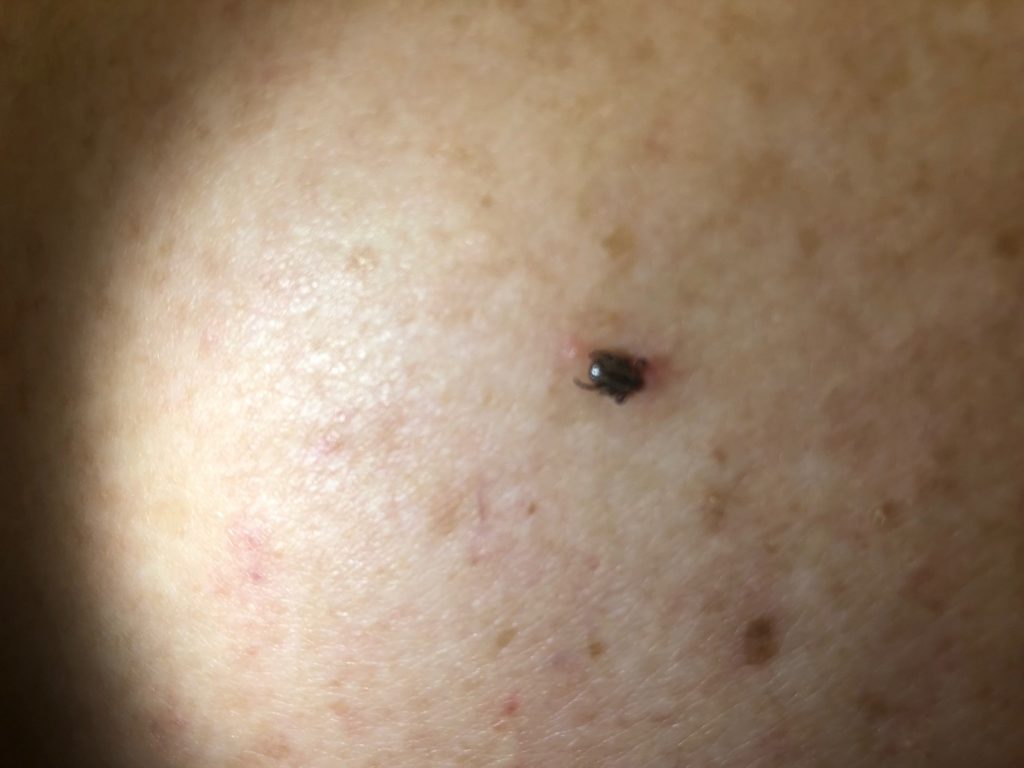
Figure 1. A 6 x 4 mm black papule.
A variety of tick removal approaches have been recommended, including:
- The tweezer removal technique—grasp tick around the mouthparts closest to the skin with tweezers and directly pull away from skin
- The card-detachment technique—entails sliding the tick body between the aperture of the device and lifting from skin for removal
- The lassoing technique—a thin filament is used to surround the tick and tightened around the body for extraction9
- The freezing technique—application of liquid nitrogen for approximately 20 seconds11
The most efficient tick-detachment method is the tweezer removal technique, which ensures complete removal of the organism. Fine tipped forceps should be used to grasp the tick as close as possible to the skin and pulled upwards with a smooth force, taking care not to twist or jerk the tick.10 (See Figure 1.) Ixodid ticks are particularly challenging to remove because their mouthparts are cemented to the skin. Pieces of the tick are easily left behind during this procedure. Residual mouth parts may lead to chronic irritation or tick-bite granuloma.9
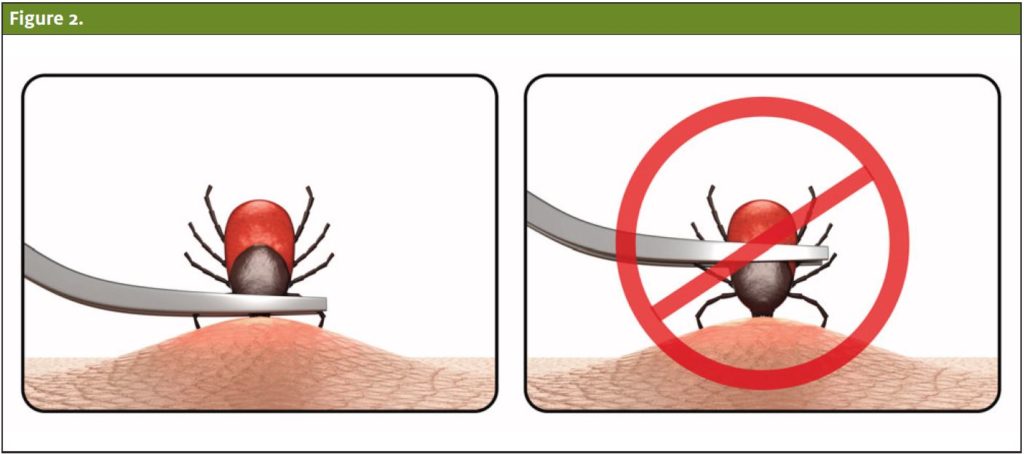
Petroleum jelly, alcohol, or fingernail polish should not be used to aid in removal, as this complicates the process of grasping and removing the tick. Heating the end of the tick with a match or electrodessication is not recommended as the heated tick contents expand and inject more material into the host. Furthermore, rotating the tick provides no additional benefit.12 If unable to successfully remove the tick with forceps, antibiotic prophylaxis may be indicated.10,13,14
In North America, the two most common families of ticks are Ixodidae and Argasidae. Ixodidae ticks are hard ticks, while the Argasidae ticks are soft ticks. A few common tick-borne illnesses include Lyme Disease, Rocky Mountain Spotted Fever, Tularemia, and Ehrlichiosis. Dermacentor variabilis specifically serves as a vector of Rocky Mountain spotted fever (RMSF) and Tularemia.3 RMSF is caused by Rickettsia rickettsii and is the most commonly reported tick-borne rickettsial disease in the U.S. It typically presents with mild constitutional symptoms and a maculopapular rash, which may extend to the palms and soles. Symptoms usually resolve within 48 hours of antibiotic treatment.15 Tularemia is caused by Francisella tularensis,which is most commonly transmitted via tick bite. Symptoms typically manifest within a few days of the tick bite and may include a variety of symptoms involving the lymph nodes, eyes, oropharynx, or respiratory system. Tularemia can be treated with antibiotics in most cases.16Dermacentor variabilis is also a common vector of tick paralysis, especially in the Southeast US.3 Tick paralysis presents as ascending paralysis due to neurotoxin release, which impairs the function of voltage-gated sodium channels. Case studies demonstrate that tick removal leads to resolution of the paralysis within hours.17
Citation: Harrington H, Pearlman R, Brodell, R. A New-Onset, Suspicious Skin Lesion. J Urgent Care Med. October 2020. Available at: https://www.jucm.com/a-new-onset-suspicious-skin-lesion/
References
- Kallini JR, Khachemoune A. Ticks and Tick bites presenting as “funny moles”: a review of different presentations and a focus on tick-borne diseases. J Clin Aesthet Dermatol. 2017;10(3):46–50.
- Akbas H, Hokelek M, Guneren E, et al. Can a tick mimic a pigmented skin lesion or melanoma? Ann Plast Surg. 2001;47(3):349–350.
- Goddard J, Layton B. A Guide to Ticks of Mississippi. Mississippi Agricultural & Forestry Experiment Station. September 2006. Available at: https://www.mafes.msstate.edu/publications/bulletins/b1150.pdf. Accessed August 21, 2020.
- Rahlenbeck S, Fingerle V, Doggett S. Prevention of tick-borne diseases: an overview. Br J Gen Pract. 2016;66(650):492–494.
- Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis. 2018;9:535–542.
- Cook MJ. Lyme borreliosis: a review of data on transmission time after tick attachment. Int J Gen Med. 2014;8:1.
- Due C, Fox W, Medlock JM, et al. Tick bite prevention and tick removal. BMJ. 2013;347:f7123.
- Sood SK, Salzman MB, Johnson BJ, et al. Duration of tick attachment as a predictor of the risk of Lyme disease in an area in which Lyme disease is endemic. J Infect Dis. 1997;175(4), 996-999.
- Akin Belli A, Dervis E, Kar S, et al. Revisiting detachment techniques in human-biting ticks. J Am Acad Dermatol. 2016;75:393–397.
- Habif TP. Clinical Dermatology: a Color Guide to Diagnosis and Therapy. St. Louis, MO: Elsevier; 2016.
- Pavlovic M, Alakeel A, Frances C. Tick removal with liquid nitrogen. JAMA Dermatol. 2013;149(5):633.
- Oteo JA, Martínez de Artola V, Gómez-Cadiñanos R, et al. Evaluation of methods of tick removal in human ixodidiasis. Rev Clin Esp. 1996;196:584–587.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Pub Health. 2015;62(5):388-392.
- Masters EJ, Olson GS, Weiner SJ, Paddock CD. Rocky Mountain spotted fever: a clinician’s dilemma. Arch Intern Med. 2003;163(7):769-774.
- McQuiston JH, Zemtsova G, Perniciaro J, et al. Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis. 2012;12(12):1059–1061.
- Gayle A, Ringdahl E. Tick-borne diseases. Am Fam Phys. 2001;64(3):461–466.
- Felz MW, Swift TR, Hobbs W. Tick Paralysis in the United States: A Photographic Review. Arch Neurol. 2000;57(7):1071–1072.
Author affiliations: Haley Harrington, Louisiana State University Health Sciences Center, Shreveport, LA. Ross L. Pearlman, MD,University of Mississippi Medical Center, Jackson, MS. Robert T. Brodell, MD, University of Mississippi Medical Center, Jackson, MS. Ms. Harrington and Dr. Pearlman have no relevant financial relationships with any commercial interests. Dr. Brodell discloses the following potential conflicts of interest: Multicenter Clinical Trials: Galderma Laboratories, L.P. – Principal Investigator; Novartis Principal Investigator; and GlaxoSmithKline – Principal Investigator. There are no conflicts of interest related to employment, stock ownership, expert testimony, grants, patents filed, received, pending, or in preparation, or royalties.
