Urgent message: In the absence of controlled outpatient trials, the author proposes urgent-care specific guidelines for treatment of community-acquired MRSA, informed by clinical experience and local and regional case reports.
Michael Dickey, MD
The goal of any treatment is to maximize the chance of a positive outcome for a patient. The purpose of a treatment guideline is to maximize the chance of positive outcomes in groups of patients that present with a similar disease states.
While there remain “many ways to skin a cat,” the theory behind the use of treatment guidelines in the primary care specialties and subspecialties including urgent care—is not necessarily complete uniformity of treatment, but to assure that treatment is consistent with available evidence from the medical literature.
The best treatment plan utilizes those treatment options that appear to show higher cure rates and shorter treatment intervals.
BACKGROUND
Ideally, we would have multiple large, controlled, community-based outpatient trials comparing various treatment options and combinations of treatments for community- acquired methicillin-resistant Staphylococcus aureus (CA- MRSA).
In the imperfect world of clinical medical practice, how- ever, we do not always have perfect evidence to rely on.
The reality is that, to date, there is little controlled re- search on the treatment of CA- MRSA, probably owing much to the recent genesis of this problem, but also due to the difficulty of controlled trials on such a genetically diverse disease agent as CA-MRSA. Some of the most useful clinical information available at present comes from case reports.
This proposed treatment guideline is also influenced by the author’s observations during treatment of approximately 1,200 cases of skin and soft tissue infections (SSTIs), of which approximately 85% were CA-MRSA, during a five-year interval (2002-2007).
A review of available literature reveals diversity of opinion on the treatment of CA-MRSA SSTIs, particularly when it comes to appropriate use of antibiotics. The diversity is so great that it leaves the impression that many regions of the country may well be dealing with less virulent strains of CA-MRSA or are just now beginning to see the problem. A literature review confirms that there is significant diversity of CA-MRSA phenotypes, and widely different prevalence rates of CA-MRSA depending on region of the U.S.1-3 Some have even advocated against the routine use of antibiotics to treat most cases of CA-MRSA SSTIs, arguing that incision and drainage is usually adequate therapy.4,5 Others have been more cautious and note that even when abscesses are treated with antibiotics showing invitro resistance, they usually get better.6
The increased virulence of CA-MRSA strains appears linked to factors such as a shorter doubling times and the Panton-Valentine leukocidin (PVL) toxin, rarely identified in healthcare-associated MRSA (HA-MRSA) isolates.7,8 This increased virulence of CA-MRSA sets it apart clinically from methicillin-sensitive S aureus and the primarily opportunistic HA-MRSA.
These distinguishing clinical features are:
- rapid or explosive growth
- large cellulitis area
- associated fever
- increased malaise, myalgia, and/or arthralgias
- toxic appearance or
However, the clinical presentation of a CA-MRSA infection is often indistinguishable from other causes of SSTI.7 While it is important for providers in endemic areas to be aware that the vast majority of the SSTIs that we see today are MRSA, we also must be cautious to remember that SSTIs can still be caused by other organisms, as well. Other organisms responsible for SSTIs include the relatively common Group A Streptococcus (GAS) (including more severe necrotizing fascitis), as well as Haemophilus influenza, Aeromonas hydrophilia (fresh water-exposed wounds), Pasteurella multocida (from animal bites), Group B, C, G Streptococcus, and, rarely, pneumo, cocci and Escherichia coli.
In addition, patients who are immunocompromised with granulocytopenia (e.g., transplant recipients and chemotherapy patients) may develop cellulitis due to gram negative bacilli such as Citrobacter, Enterobacter, Pseudo- monas, Proteus, and Serratia. Thus, providers should continue to culture wounds for confirmation of pathogen identification whenever possible.4
PRIMARY TREATMENT PRINCIPLES FOR SSTIs AT RISK FOR CA-MRSA
In essence, there are three primary principles for treatment of SSTIs at risk for CA-MRSA:
- Thorough and complete wound debridement and maintenance of debrided state
- Aggressive multi-drug antibiotic treatment
- Treatment of underlying comorbid factors, e.g., diabetes and edema states affecting venous return
We will break down each of these principles further.
Wound Debridement
Anesthesia
Good wound debridement begins (and ends) with adequate anesthesia. In general, this is a matter of a good field infiltration (Figure 1). A good infiltration over a large abscess can take several minutes to obtain. The use of multiple drugs (e.g., bupivacaine, lidocaine, and epinephrine) often makes for more complete and durable anesthesia. If adequate anesthesia cannot be obtained in the out patient setting, the patient should be immediately referred for operating room surgical debridement under regional or general anesthesia.
For pediatric cases or very apprehensive patients, a mixture of lidocaine and prilocaine or an occlusive dressing of viscous lidocaine for 30 minutes to one hour prior to field infiltration may be beneficial.
Also, topical viscous lidocaine used to moisten packing and placed inside abscess cavities prior to subsequent wound care appears to be a useful adjunct for reducing discomfort and allowing for adequate wound irrigation and/or cleaning.
Incision and Drainage
Adequate exposure of the abscess cavity is likely the most critical aspect of good drainage and continued care of an abscess.
One of the greatest obstacles to clearing an infection is reformation of abscess and/or tunneling of the infection through subcutaneous tissue or into deeper structures. The primary wound incision must be large enough to allow adequate wound care and inspection to prevent formation of additional abscesses.
The primary incision length over an abscess should in most cases approach one half the diameter of the abscess (e.g., a 2 cm abscess should have a 1 cm incision).
In the author’s experience, the vast majority of abscesses can be treated adequately through incisions measuring 1 cm to 3 cm. No incision into an abscess should be smaller than 8 mm to 10 mm. Incisions any smaller than this do not allow for adequate wound care and drainage. Deep abscesses need a proportionately larger incision in order to maintain adequate drainage. Abscesses that are beneath 3 cm to 4 cm of subcutaneous tissue do best with incisions that approach their full diameter. Wounds should be thoroughly probed with a hemostat or similar instrument with an effort to coalesce the abscess into a single well confluent cavity. The wound cavity should then be irrigated copiously with sterile saline or water. Either a drain or packing must be placed for the initial 24 hours following drainage. Plain packing, normal saline wet to dry, iodoform gauze or Penrose drain are all suitable. Strong consideration should be given to using a drain instead of packing in fistulous tracks.
Often, a more expedited sterilization of a wound can be obtained by making a second incision into the distal end of a subcutaneous track and running the drain out of both ends of the tract.
These wounds should be treated twice daily with nor- mal saline wet-to-dry dressing changes or cleanings twice daily with saline or hydrogen peroxide and cotton swabs in order to maintain adequate wound debridement.
Wound packing should not be left in a wound for an extended period of time. Packing left as long as 48 hours in the wound appears to foster formation of second abscesses.
This does not apply to drains, however; usually, drains should be left until drainage is minimal and the cellulitis component of the infection is significantly improved. The area around the drain should be cleared of debris with saline or peroxide at least once daily to maintain adequate drain function as long as the drain is in place. With certainty, inadequate drainage of the CA-MRSA SSTI(s) appears to be a significant cause for treatment resistance and treatment failure. However, as large SSTIs with a significant cellulitis component are the rule and these areas of cellulitis routinely produce satellite abscesses, it seems unlikely that drainage of the abscess alone is an appropriate empiric treatment of any but the smallest and most superficial lesions.
The Centers for Disease Control and Prevention continues to recommend the routine culture of all abscesses, even in areas with epidemic outbreaks of CA- MRSA. The CDC’s Summary of Experts Meeting on MRSA in March of 2006 rationalized this by stating that “obtaining cultures of purulent skin and soft tissue infections is still important to monitor trends in suscep- tibility of S aureus to non beta-lactam agents.”4
Antibiotic Treatment
The area of greatest disagreement in the treatment of SSTI is the use of antibiotics. Possible causes for such divergent opinions include geographic variations in frequency of CA-MRSA isolation, susceptibility patterns, and varia- tions in virulence, as well as deep-seated disagreements on the use of certain classes of antibiotics in infections that are viewed by some as less serious or non life-threat- ening. It is possible that recent national press coverage of this disease may have an impact on those biases.
While some sources have recently advocated a “trial of incision and drainage” for CA-MRSA abscesses “smaller than 5 cm,”9 this approach does not appear to take into account the geographic variability of the prevalence of MRSA (20% to 90%), the increased virulence of CA-MRSA in certain endemic areas, and unpredictable patient follow-up in urgent care and emergency medicine settings.
Often, in these endemic areas, many (if not most) patients present with a rapidly progressive cellulitis and/or explosive growth in abscess size. The common observa- tion of significant growth of the area of cellulitis 24 to 48 hours after appropriate incision and drainage argues strongly for routine use of antibiotics.
The author speculates that those who argue against the routine use of antibiotics are from regions that are still seeing virulence more akin to HA-MRSA infections or less virulent strains of CA-MRSA.
The principles of CA-MRSA antibiotic treatment being proposed here include:
- frequent use of antibiotic combinations from the onset of treatment
- aggressive dosing of certain antibiotics (e.g., TMP- SMX)
- early consideration of “second-line drugs,” including intravenous vancomycin
- avoidance of drugs likely to have resistance or that are prone to develop resistance during treatment (i.e. avoidance of B-lactams, macrolides and older quinolones, D disk testing for inducible clindamycin resistance).
Following is a brief overview of the currently available classes of antibiotics for treatment of CA-MRSA SSTIs (Table 1).
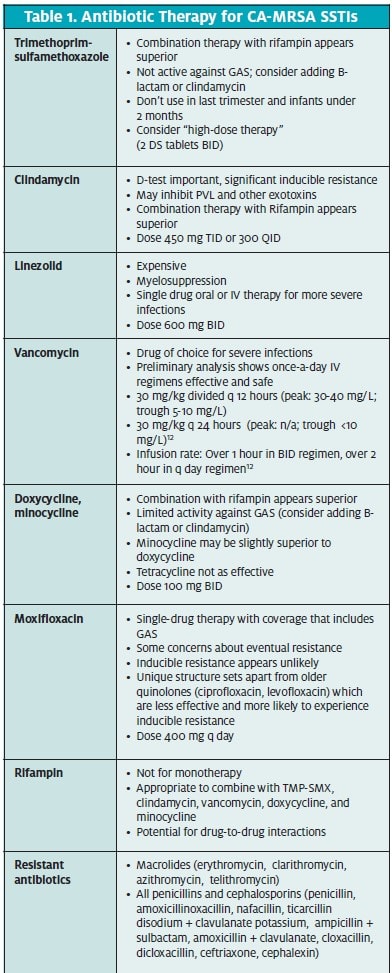
Vancomycin has been used to treat serious MRSA infections for the last 15 to 20 years and remains the gold standard for treating MRSA. However, despite a high in vitro sensitivity, treatment failure rates in the 40% range with single-drug therapy of serious infections are reported.10,11,12 Combination therapy with rifampin improves response rates. Once daily intravenous therapy, while not currently in widespread use, makes for more feasible outpatient therapy in the urgent care setting.12 Further studies into the efficacy of once-daily vancomycin are warranted.
Clindamycin is FDA-approved for the treatment of serious infections due to S aureus and has been used suc- cessfully to treat CA-MRSA. However, inducible clin- damycin resistance is an issue in erythromycin-resist- ant, clindamycin-sensitive S aureus isolates.13 Inducible clindamycin resistance can be detected through a spe- cialized laboratory test called the D-zone test.14
Clindamycin appears to exhibit a unique inhibition of the PVL toxin, which may be of significant benefit in the inhibition of further cellulitis and abscess spread.15 Another very important benefit to adding clindamycin to any regimen for treatment of SSTI is the addition of good-to-excellent coverage for GAS. Neither SMP-TMP nor doxycycline has adequate coverage for GAS.
Clostridium difficile-associated diarrhea (CDAD) may occur more frequently with clindamycin compared with other antibiotics commonly used to treat CA- MRSA; however, it is still a relatively rare complication of treatment or CA-MRSA, even with the use of clin- damycin.
Tetracycline (specifically, doxycycline) is also FDA- approved for the treatment of S aureus skin infections. The prevalence of tetracycline resistance in CA-MRSA remains low.16
Further, much of the reported resistance to tetracycline is due to the tetK gene, which only confers resistance to tetracycline specifically; it does not confer resistance to doxycycline or minocycline. Replacement of tetracycline with doxycycline or minocycline on susceptibility testing may be desirable in the future, particularly if the prevalence of tetracycline resistance increases.
In a recent case series, the long-acting tetracyclines (doxycycline and minocycline) performed well for the treatment of MRSA SSTIs caused by tetracycline-sus- ceptible isolates.16
The tetracyclines are not recommended during pregnancy or for children under the age of 8. In addition, as group A Streptococcus infections are also an important cause of SSTIs, it is important to remember that significant resistance to tetracycline is common in group A Streptococcus isolates.
Trimethoprim-sulfamethoxazole
(TMP-SMX) is not FDA-approved for the treatment of any form of staphylococcal infection. However, TMPSMX is “rapidly bactericidal against MRSA in vitro compared with most other orally available antimicrobials.”17 There are also a number of case reports reporting successful use of TM- SMX in the treatment of S aureus infections, including MRSA. One case report describes the use of “high dose” (oral TMP 20 mg/kg/day SMX 100mg/kg/day) for the treatment multi-drug resistant S aureus infected orthope- dic implants. Treatment periods were six to nine months, with overall success rate of 66.7%.18
Nonetheless, in clinical practice drug treatment fail- ure remains an issue for TMP-SMX. Combination with rifampin appears to improve responses to treatment.19-21 Also, it is clinically important to remember that GAS is another common cause of SSTIs, and GAS is usually re- sistant to TMP-SMX.
Additional coverage, such as clindamycin, should be considered to cover any SSTI until cultures have shown that GAS is not responsible for the infection.4
In Central Texas, TMP-SMX is often given as a pre- ferred choice to treat SSTI abscesses by lecturers giving presentations to emergency medicine, urgent care, and primary care physicians. Antibiograms would seem to support this recommendation.
However, in clinical practice, we have found an extraordinarily high failure rate with standard doses of TMP-SMX alone. The addition of rifampin +/- clindamycin appears to substantially improve success rates. Granted, this is vague and only anecdotal information, but our experience would speak strongly against TMP-SMX monotherapy in any abscess with signifi- cant overlying cellulitis, near joints, in the perineal area, and near facial structures. Also of concern is that GAS infections are another important cause of SSTIs and are resistant to TMP-SMX therapy.
TMP-SMX should not be used in children under 2 months of age or in women in the last trimester of pregnancy.
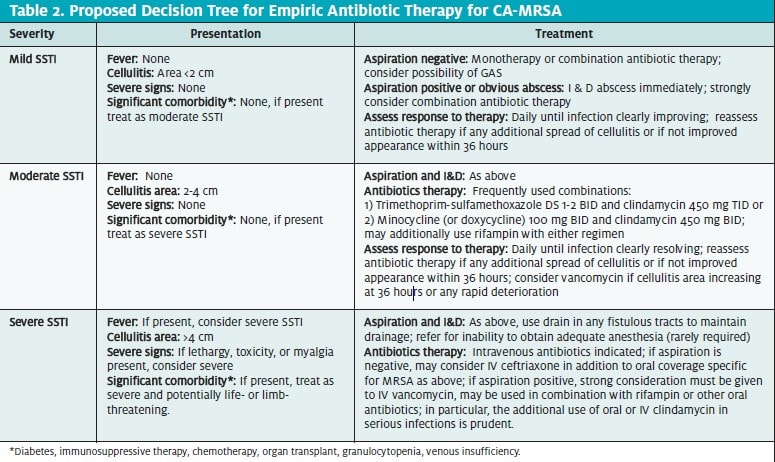
Quinolones: Conversely, the CDC notes that fluo- roquinolones and macrolides “are not optimal choices for empiric treatment of community-associated SSTI(s) possibly caused by S aureus…because of a relatively high prevalence of resistance among S aureus isolates in the community or the potential for rapid development of resistance.”4
This statement bears further analysis, however.
Because of frequent resistance, macrolides are clearly not an appropriate therapeutic choice for treating SSTIs due to MRSA. However, in reviewing the literature, it would seem that the CDC—with input from the “expert panel”may be overstating the case against the use of certain fluoroquinolones.
Currently, in many regions of the United States, the rates of CA-MRSA resistance to quinolones remain low. There is concern that this rate appears to be increasing, however.
While it is also true that older quinolones, such as ciprofloxacin, are prone to inducible resistance—partic- ularly with S aureus22,23—this does not appear to be the case with newer C8 modified quinolones such as mox- ifloxacin and garenoxacin, which has yet to be ap- proved in the U.S.24-26 Because the minimum inhibitory concentrations of the newer quinolones are lower than those of the older quinolones (ciprofloxacin and lev- ofloxacin), there is less chance for inducible resistance to develop.
However, it should be remembered that quinolone resistance is primarily class specific. As CA-MRSA quinolone resistance increases, the newer modified quinolones may become less effective. In spite of these theoretical concerns, it is far from a foregone conclusion that the use of moxifloxacin now to treat SSTIs will re- sult in a more rapid antibiotic resistance than the use of any of the other treatment options currently available.
Linezolid, first released in 2000, is active against both HA-MRSA and CA-MRSA and has recently found increased use in the treatment of endemic outbreaks of CA-MRSA infections.
Some studies have shown the effectiveness of line zolid to approach that of vancomycin in the treatment of MRSA.27,28 Linezolid, like clindamycin, has an in- hibitory effect on the production of PVL toxin by S aureus.15 The main limiting factor for the use of line zolid is the cost of $130/day.
Adverse effects of linezolid include myelosuppression, neuropathy, and a particularly high risk of drug in- teraction with selective serotonin reuptake inhibitors re- sulting in serotonin syndrome.
Rifampin has long been used as to treat tuberculosis in combination with other medications and is most familiar to clinicians for this use. Although rifampin shows high sensitivities for CA-MRSA, effective cure rates are low when it is used as single-drug therapy. This is at least partially due to the fact that when rifampin is used as a single agent, S aureus appears to develop re- sistance rapidly.29
However, numerous studies have shown that when ri- fampin is used in combination with certain other antimicrobials, cure rates are improved substantially.19,21,27 In particular, combinations with vancomycin, trimetho primsulfamethoxazole (trimeth/sulfa), and minocycline appear to improve clinical outcomes.
Studies of the combined use of linezolid and rifampin showed significant disagreement, but as a whole tended to indicate a lack of antagonism between the two antibiotics, while showing evidence of less induced resistance to rifampin; several studies indicated synergy when using these antibiotics in combination.27,30
Rifampin does appear to exhibit synergy with the older quinolones, particularly in reducing inducible resistant. However, we are unaware of any studies addressing possible combination with the newer quinolones in the treatment of CAMRSA.
Because of the high observed failure rate of single drug therapy at our facility, we have instituted the following policy:
- Mandatory Use of Combination Therapy for CA-MRSA
All patients being treated empirically or with a clinical diagnosis or SSTI due or possibly due to CAMRSA are to be placed on combination therapy using rifampin and/or clindamycin in addition to one or more of the following: vancomycin, linezolid, trimeth-sulfa, or tetracycline (minocycline, doxycycline). If, in the physician’s judgment, there is contraindication to this combination therapy, the rationale for withholding combination therapy must be documented in the patient chart. Alternative appropriate monotherapy includes linezolid or possibly moxifloxacin. Vancomycin should be considered appropriate as either monotherapy or in combination in most serious SSTI.
IN CONSIDERATION OF GAS
Group A Streptococcus (GAS) is also an important cause of SSTI. In particular, wounds with predominantly cellulitis or impetigo appearance should be considered possibly due to GAS. Tetracyclines and TMP-SMX are not adequate treatments for suspected GAS infections. Appropriate coverage for GAS includes B-lac- tams, macrolides or clindamycin.
COMMUNITY RESISTANCE PATTERNS
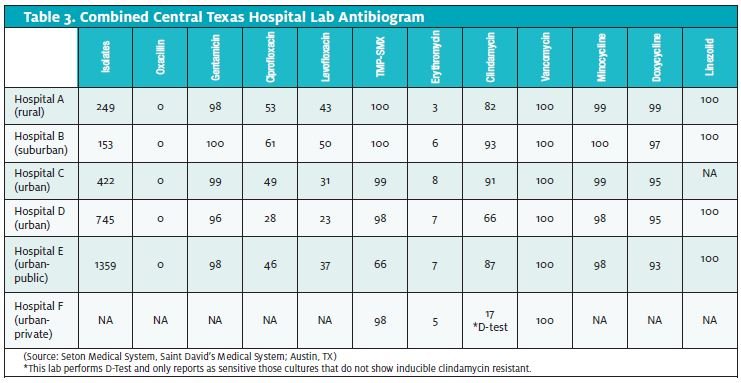
As the prevalence, virulence, and sensitivities of CA-MRSA vary signif- icantly from region to region, it can be helpful to obtain local antibi- ogram data. Unfortunately, lab antibiograms routinely combine data from CA-MRSA and HA-MRSA.
Some useful information can still be gleaned by studying community resistance patterns. The diverse phenotypes of these two broad classifications of S aureus make it difficult to distinguish them definitively in the laboratory.
In Table 3, note the low sensitivities to clindamycin in the only lab- oratory doing the D-Test for inducible clindamycin resistance. Certainly, without that information, clindamycin would appear to be much more effective than it actually is likely to be in this particular geographic region.
Also, note the falling sensitivities to TMP-SMX when progressing from rural to more urban hospitals. This may reflect a higher percent- age of HAMRSA isolates.
TREATMENT OF UNDERLYING COMORBID FACTORS
Factors regarding certain comorbid conditions bear mention.
Diabetic patients require close monitoring of their glucose measurements during treatment. Infection can predispose these patients to worsening hyperglycemia, making treatment more difficult. Ketosisprone diabetics are at risk for developing diabetic ketosis. Many, if not most, diabetic patients will require additional insulin, modification of oral regimen, or initiation of temporary insulin therapy during trea ment.
Edema states affecting the area of infection can make for very difficult eradication of infection. Therapies including elevation, sequential compression, or graduated compression to affected edematous areas are needed to improve venous return.
CONCLUSION
Failed outpatient therapy is a significant problem in the management of CAMRSA. Inadequate initial incision and drainage, inadequate wound management after initial I & D, and inadequate antibiotic coverage are potential causes of failed outpatient therapy.
Increased provider attention to these critical aspects of treatment should result in reduced numbers of prolonged outpatient treatment and reduced numbers of outpatient treatment failure.
Currently, the medical literature is very confused on the subject of antibiotic therapy for CA-MRSA SSTIs. Those of us on the front line must continue to assess the literature carefully and with critical thought. Hopefully, as new case series are evaluated, improved evidence and consensus will result.
REFERENCES
- Styers D, Sheehan DJ, Hogan P, et Laboratory-based surveillance of current antimi- crobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2.
- Johnson LB, Saravolatz Community-acquired MRSA: Current epidemiology and man- agement issues. Infect Med. 2005;22(1):16-20.
- Fridkin SK, Hageman JC, Morrison M, et al for the Active Bacterial Core Surveillance Pro- gram of the Emerging Infections Program Methicillin-resistant Staphylococcus au- reus disease in three communities. N Engl J Med. 2005;7;352(14):1436-1444. Erratum in: N Engl J Med. 2005;352(22):2362.
- CDC—Strategies for clinical Management of MRSA in the Community: Summary of an Experts Meeting Convened by the Centers for Disease Control and Prevention, March
- Hankin A, Everett Are antibiotics necessary after incision and drainage of a cuta- neous abscess? (From the Department of Emergency Medicine, Hospital of the University of Pennsylvania.) Ann Emerg Med. 2007;50(1):49-51.
- Paydar KZ, Hansen SL, Charlebois ED, et Inappropriate antibiotic use in soft tissue infections. Arch Surg. 2006;141(9):850-854; discussion 855-856.
- Naimi TS, LeDell KH, Como-Sabetti K, et Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976- 2984.
- Vandenesch F, Naimi T, Enright MC, et Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: Worldwide emer- gence. Emerg Infect Dis. 2003:9(8):878-984.
- Lee MC, Rios AM, Aten MF, et Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123-127.
- Sakoulas G, Moise-Broder PA, Schentag J, et Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus au- reus bacteremia. J Clin Microbiol. 2004;42:2398-2402.
- Moise PA, Schentag Vancomycin treatment failures in Staphylococcus aureus lower respiratory tract infections. Int J Antimicrob Agents. 2000;16 (Suppl 1):S31-S34.
- Cohen E, Dadashev A, Drucker M, et Once-daily versus twice-daily intravenous ad- ministration of vancomycin for infections in hospitalized patients. J Antimicrob Chemother. 2002:49:155-160.
- Drinkovic E, Fuller ER, Shore KP, et Clindamycin treatment of Staphylococcus aureus expressing inducible clindamycin resistance. J Antimicrob Chemother. 2001;48: 315-316.
- CDC—Online publication APPENDIX (to routine disk diffusion test)
- Clindamycin disk induction test for Staphylococcus spp. wwwn.cdc.gov/nltn/ pdf/2004/2_Hindler_D-Test.pdf
- Dumitrescu O, Boisset S, Badiou C, et Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob Agents Chemother. 2007;51(4):1515-1519.
- Ruhe JJ, Monson T, Bradsher RW, et Tetracyclines for MRSA infections. Clin Infect Dis. 2005;40:1429-1434.
- Kaka AS, Rueda AM, Shelburne III SA, et Bactericidal activity of orally available agents against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2006;58:680–683.
- Stein A Bataille JF, Drancourt M, et Ambulatory treatment of multidrug-resistant Staphylococcus-infected orthopedic implants with high-dose oral co-trimoxazole (trimetho- prim-sulfamethoxazole). Antimicrob Agents Chemother. 1998;42(12):3086-3091.
- Varaldo PE, Debbia E, Schito In vitro activities of rifapentine and rifampin, alone and in combination with six other antibiotics, against methicillin-susceptible and methi- cillin-resistant staphylococci of different species. Antimicrob Agents Chemother. 1985;27(4):615–618.
- Hackbarth CJ, Chambers HF, Sande Serum bactericidal activity of rifampin in com- bination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother. 1986;29(4):611-613.
- Iyer S, Jones Community-acquired methicillin-resistant Staphylococcus aureus skin infection: A retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Blumberg HM, Rimland D, Carroll DJ, et Rapid development of ciprofloxacin resist- ance in methicillin-susceptible and –resistant Staphylococcus aureus. J Infect Dis. 1991;163(6):1279-1285.
- Piercy EA, Barbaro D, Luby JP, et Ciprofloxacin for methicillin-resistant Staphylococ- cus aureus infections. Antimicrob Agents Chemother. 1989;33(1):128-130.
- Dajcs JJ, Thibodeaux BA, Marquart ME, et al. Effectiveness of ciprofloxacin, lev- ofloxacin, or moxifloxacin for treatment of experimental Staphylococcus aureus keratitis. Antimicrob Agents 2004;48(6):1948-52.
- Shopsin B, Zhaob X, Kreiswirth BN, et Are the new quinolones appropriate treat- ment for community-acquired methicillin-resistant Staphylococcus aureus? Int J Antimicrob Agents. 2004;24:32–34.
- Noviello S, Ianniello F, Leone S, et Comparative activity of garenoxacin and other agents by susceptibility and time-kill testing against Staphylococcus aureus, Streptococcus pyogenes and respiratory pathogens J Antimicrob Chemother. 2003;52: 869–872.
- Jacqueline C, Caillon J, Le Mabecque V, et In vitro activity of linezolid alone and in combination with gentamicin, vancomycin or rifampicin against methicillin-resistant Staphylococcus aureus by time–kill curve methods. J Antimicrob Chemother. 2003;51(4):857- 864. Epub 2003 Mar 13.
- Weigelt J, Itani K, Stevens D, et Linezolid versus vancomycin in treatment of com- plicated skin and soft tissue infections. Antimicrob Agents Chemother. 2005;49(6):2260- 2266.
- Strausbaugh LJ, Jacobson C, Sewell DL, et Antimicrobial therapy for methicillin-re- sistant Staphylococcus aureus colonization in residents and staff of a Veterans Affairs nurs- ing home care unit. Infect Control HospEpidemiol. 1992;13(3):151-159.
- Grohs P, Kitzis M-D, Gutmann L. In vitro bactericidal activities of linezolid in combina- tion with vancomycin, gentamicin, ciprofloxacin, fusidic acid, and rifampin against Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47(1):418-420.
