Published on
Urgent message: Patients with glucose levels either too high or too low often require immediate, potentially life-saving interventions in the urgent care setting. These patients are often found to be diabetic.
Allan F. Moore, MD, Nicolas Abourizk, MD, Jeffrey Collins, MD, MA Diabetes is a common chronic disease affecting approximately 7% of the United States population. Of these individuals, 17.5 million carry a diagnosis of diabetes and over 6 million are undiagnosed. An estimated 54 million additional Americans have prediabetes.
In 2007, the total annual economic cost of diabetes care in the U.S. was estimated at $174 billion—with the majority of this cost being spent on urgent and emergent care and in-patient hospitalization.1
Unfortunately, due to a multiple of factors (e.g., primary care and subspecialty access, insurance resources, the level of patient understanding about their condition), diabetes care is often fragmentary or insufficient. Hence, diabetic patients will continue to seek care in walk-in centers, and the likelihood of encountering serious diabetic complications in urgent care will increase.
Common glycemic emergencies seen in diabetic patients in the urgent care setting include diabetic ketoacidosism (DKA), hyperglycemic hyperosmolar state (HHS), and hypoglycemia. All three require immediate evaluation and treatment.
This review will take a case-study approach to exemplify the immediate triage, evaluation, and treatment of adult patients with glycemic perturbations.
Case Studies: Patient 1
Presentation
C.K. is a 19-year-old female who presents to the urgent care with her mother. She had been feeling weak and tired for several days but now, according to her mother, is not eating. She has been vomiting “on and off.” Her mother states “she’s not herself.”
In triage, we find:
- oral temperature 4°F
- pulse 112
- BP 84/50 mmHg
The patient is ill-appearing and states her stomach hurts. A screening urine dip reveals:
- 3+ WBC
- 2+ RBC
- • + nitrite
- 1+ protein
- pH 0
- 030 specific gravity
- large ketones
- large glucose
- urine HCG is negative
- fingerstick glucometer reads >600 mg/dL
The patient is brought back to an examination room immediately.
Discussion
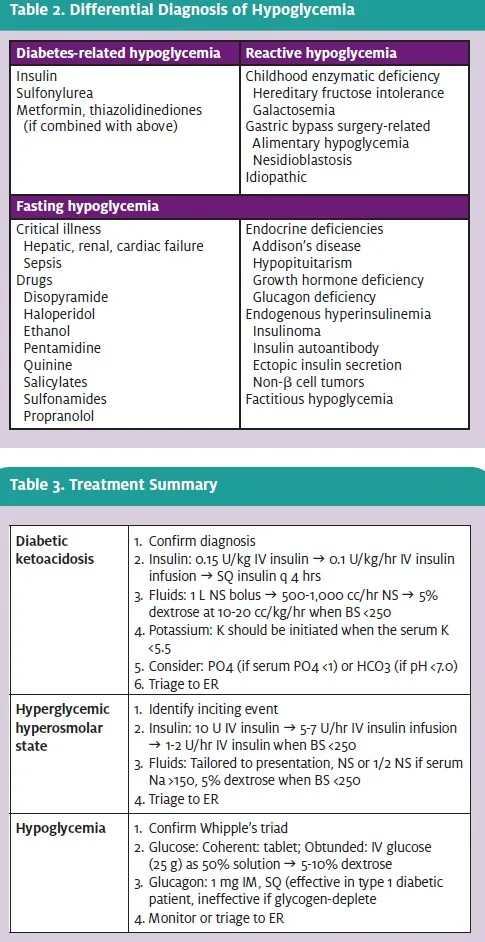
This patient presents with signs, symptoms, and lab- oratory testing diagnostic of DKA, a potentially life- threatening condition with a mortality of approximately 5%.2 Although commonly associated with type 1 diabetes, DKA is seen in patents with type 2 dis- ease as well, especially obese African-Americans. On average, patients with type 1 diabetes will have one episode of DKA in their lifetime, accounting for approximately 100,000 admissions annually in the U.S.2 The diagnosis of DKA requires an understanding of both the clinical and laboratory derangements associated with the condition. Patients with DKA are uniformly volume-depleted with dry mucous membranes, decreased jugular venous pressure, orthostatic hypotension, tachycardia, and oliguria.
Acetone production produces a fruity odor on the patient’s breath, and respirations may be deep and rapid (Kussmaul breathing), a response by the medullary respiratory center to worsening acidosis. A peculiar and poorly understood clinical feature of DKA is severe abdominal pain, especially in children, which has been confused with an acute surgical abdomen.3 The etiology of the abdominal pain is suspected to be a combination of electrolyte derangement, dehydration, and acidosis, although other authors suggest hepatic enlargement and stretching of Glisson’s capsule may also be involved.
The biochemical derangements of DKA include an inter-related triad of hyperglycemia (blood glucose >250 mg/dL), acidosis (arterial pH <7.3), and ketonemia (anion gap >14). The severity of DKA is not reliably predicted by the level of hyperglycemia and requires integration of clinical and laboratory findings. Although an anion gap metabolic acidosis is the most common acid-base disturbance on presentation, a pure hyperchloremic metabolic acidosis or combination of the two disorders can also be seen.
Other common laboratory findings on presentation include hyperkalemia and hyperphosphatemia which result from acidosis and insulin deficiency, forcing potassium and phosphate out of the intra-cellular compartment into the extra-cellular compartment. Hyponatremia results as water follows electrolyte movement into the extracellular space. Leukocytosis, hyperlipidemia, and hyperamylasemia are also common laboratory findings.
Because up to 20% of DKA cases involve patients not known to be diabetic, a broad differential diagnosis must be considered. Profound hyperglycemia may be seen in HHS (discussed later in this article), and stress hyperglycemia as- sociated with burns and other severe injuries. Ketosis may be seen in alcoholic ketoacidosis, a result of binge drinking in a chronic alcoholic patient that can be distinguished from DKA via an elevated [3-hydroxybutyrate to acetoacetate ratio, as well as starvation keto- sis, a condition resulting from fasting for at least 24 to 48 hours, which presents as mild ketosis (bicarbonate >18 mEq/L) in the absence of hyperglycemia.
A number of conditions may result in an anion gap acidosis, including lactic acidosis, renal failure, and in- gestions of salicylate, methanol, ethylene glycol, and paraldehyde.4
Treatment
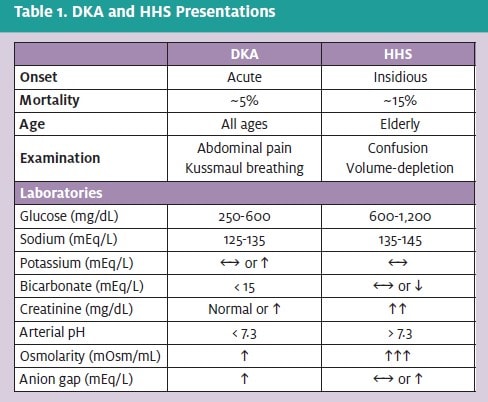
Emergent therapy for DKA must be rapid. Intravenous (IV) fluids and insulin therapy are the first considerations. Although there has been controversy concerning the optimal resuscitation fluid, these authors prefer normal saline. Following a bolus of 1 liter of normal saline (0.9% NaCl), the infusion should be maintained at a rate of 500 mL/hr to 1,000 mL/hr for the next two hours. Once the serum blood glucose decreases to 250 mg/dL, dextrose 5% should be added to the replacement fluids in order to avoid hypoglycemia or cerebral edema.
Intravenous fluids should then be administered at 10 mL/kg/hr to 20 mL/kg/hr until the patient is hemodynamically stable and finally titrated to match urine output. Typically, intravenous fluids are needed for at least 48 hours, and care should be taken in patients with renal dysfunction, congestive heart failure, or other conditions with impaired fluid homeostasis.
Intravenous administration of insulin is preferred to intramuscular or subcutaneous dosing, as the IV route results in larger reductions in serum glucose and ketone levels in the first two hours following presentation. An initial IV bolus of 0.15 U/kg of regular insulin is first administrated followed by a constant IV infusion of 0.1 U/kg/hr. The rate is reduced when the serum glucose reaches 250 mg/dL, at which time dextrose 5% is added to the replacement fluid.
Intravenous insulin should continue until the an- ion gap and ketosis have resolved. Generally, most urgent care practices would transfer the patient to a hospital emergency room at this point; however, if unable to do so, follow this algorithm.
Optimal insulin titration results in an hourly serum glucose reduction of between 50 mg/dL and 70 mg/dL. Once DKA has resolved (pH 7.3, bicarbonate>18 mEq/L, serum osmolarity <200 mOsm/kg), insulin injections every four hours are initiated. Known diabetics can resume their prior insulin regimens; newly diagnosed diabetic patients require, on average, 0.6 U/kg/day in divided doses. Insulin titration is of- ten required again after discharge once the insulin resistance associated with the DKA state has resolved. Some overlap in IV and SQ insulin dosing ensures that ketosis and hyperglycemia do not recur. Another important consideration for the emergent therapy of DKA includes timely electrolyte monitor- ing and replacement.
Most DKA patients are hyperkalemic on presentation due the extra-cellular shift of potassium out of cells during acidemia and insulin deficiency, despite total body potassium deficiency (which typically ranges from 500 mEq/L to 700 mEq/L).
However, as many as 10% of DKA patients may be hypokalemic on presentation. As insulin and fluids are administered, potassium levels often drop precipitously as potassium re-enters cells. Potassium replacement, either as potassium phosphate or potassium acetate, should be initiated when the serum potassium level falls below 5.5 mEq/L. Potassium should not be given at a rate greater than 40 mEq/hr, and plasma levels should remain between 4 mEq/L and 5 mEq/L during replacement.
Electrocardiogram monitoring may be required if large amounts of potassium are needed. Phosphate dysregulation mirrors potassium dysregulation, with phosphate exiting cells during acidosis and insulin deficiency, and returning during insulin and fluid replacement.
Phosphate replacement is not required unless the serum phosphate level falls below 1 mEq/L or the patient is hypoxic or anemic. If required, 20 mEq/L to 30 mEq/L of potassium phosphate can be added to replacement fluids. Hypocalcemia may result and should be monitored. Bicarbonate replacement is usually not required, and has generally not been shown to be effective. Our standard practice is to administer bicarbonate replacement only in cases of life-threatening hyperkalemia or severe academia (pH <7.0); however, this approach remains unproven.5
Finally, it is critical to identify the precipitating event, triage the patient appropriately, and implement future prevention strategies. The most common precipitating factors for DKA include infection (our patient in Case 1 had pyelonephritis), cardiovascular events, medical non-adherence due to psychosocial reasons, pump failure, other medical illnesses, and carbohydrate-altering medications.
Most DKA patients will require at least a brief inpatient admission, and those with hypotension, oliguria, mental obtundation, or coma require intensive care admission and observation. Several excellent reviews of DKA diagnosis and pathophysiology are currently available.6-8
Case Studies: Patient 2
Presentation
A.G. is a 64-year-old female brought into the urgent care center by her two daughters, who state they went to visit her this morning and found her lying on the sofa seeming “very tired.” The daughters tell you their mother has type 2 diabetes, high blood pressure, and “heart trouble.” They are unsure of her medicines and have not brought them with her. The patient is not responding to questions in triage and is brought back to an exam room, where you find:
- temperature 96.4° F
- heart rate 58
- BP 96/65 mmHg
You are awaiting a urine sample. A fingerstick glucometer reads >600 mg/dL. She is responsive to your questions initially but becomes less so during the course of your examination.
Discussion
This case describes an acute hyperglycemic condition that is similar to DKA; however, ketosis—the hyperosmolar hyperglycemic state formerly known as hyperglycemic hyperosmolar nonketotic coma or hyperglycemic hyperosmolar non-ketotic state is absent.
As with DKA, there is insufficient circulating insulin and elevation in counter-regulatory hormones. Typically, patients with HHS are elderly and present with a week or more of poor fluid intake resulting in mental confusion and other neurological deficits.
Most often, the lack of oral intake is gradual over days to weeks and is associated with a serious underlying condition. Sepsis, pneumonia, and other infections are common precipitants. Medications that decrease insulin secretion or action (e.g., diuretics, beta-blockers, phenytoin [Dilantin]) and medications that cause insulin resistance (e.g., cortisol, growth hormone, thyroid hormone) may also be responsible.
Although less frequent than DKA, accounting for less than 1% of hospital admissions, mortality from HHS may be high as 15%.9 The dehydration that follows in the setting of relative insulin deficiency results in profound hyperglycemia. The subsequent osmotic dieresis worsens the volume depletion and hyperosmolarity. The hyperosmolarity, in turn, worsens the mental dysfunction. The available insulin is unable to inhibit gluconeogenesis or promote glucose uptake by peripheral tissues but is able to prevent ketosis.
The physical examination reveals hypovolemia in the absence of ketosis (no Kussmaul breathing or ace- tone-breath). Mental obtundation and coma are common findings, and focal neurological symptoms are possible. Often, patients are unable to mount a fever despite an active infection.
Laboratory abnormalities of HHS overlap greatly with DKA, as both conditions are hyperglycemic, hyperosmolar conditions. The hyperglycemia of HHS is typically more pronounced than that of DKA, with serum glucose levels commonly >1,000 mg/dL. The sodium levels are traditionally higher than in DKA, given the significant intravascular volume loss.
If corrected for the level of hyperglycemia, most HHS patients are frankly hypernatremic. Magnesium, chloride, and phosphate levels are typically normal, while bicarbonate levels are normal or mildly decreased. Renal insufficiency is more common in HHS than in DKA. Given the lack of ketone and anion produc- tion, patients usually are not acidemic, and the anion gap may be normal or slightly elevated. If ketonuria is present, it is usually secondary to starvation. Differences in DKA and HHS are reviewed in Table 1.
Treatment
Treatment for HHS focuses on the two largest derangements: volume depletion and hyperglycemia. Treatment must be approached with care, however, given these patients are usually older than DKA patients and often have comorbidities which may impair their ability to handle rapid fluid resuscitation. Once basic evaluations have been completed to identify and treat the underlying problem, 0.9% saline is given over the first few hours to remedy the volume depletion. If the serum sodium is >150 mEq/L, 0.45% saline is administered to provide free water.
Given the likely comorbidities and subacute presentation, fluid correction must be tailored for the individual patient in order to prevent rapid changes in serum sodium levels. After hemodynamic stability is achieved, the patient’s free water deficit is corrected with 5% dextrose in water. Commonly, HHS patients will be 10 liters or more deficient in free water.
Insulin is also a core component of therapy for HHS patients, although the fluid replacement described above will also significantly lower serum glucose levels. An initial bolus of 10 U of IV regular insulin followed by a constant infusion of between 5 U/hr and 7 U/hr is a reasonable initial approach.
As described previously in the treatment of DKA, dextrose should be added to the fluid replacement and the insulin rate should be decreased to between 1 U/hr and 2 U/hr when the serum glucose reaches 250 mg/dL. Patients should be transferred to a hospital ER, but if delays ensue, they can be transitioned to multiple SQ insulin injections once the serum glucose has stabilized and mental condition cleared.
Case Studies: Patient 3
Presentation
L.T. is a 54-year-old male who is brought into your urgent care center after “passing out” in the diner next door. In the examination room, you find:
- he is afebrile
- pulse 98
- BP 110/68.
The patient is pale and talking about “pancake specials.” An initial fingerstick glucometer reading is 38 mg/dL and a repeat is 34 mg/dL. A bottle of glyburide is found subsequently in a coat pocket.
Discussion
Hypoglycemia is a potentially lethal condition which, if recognized promptly, can be easily reversed. How- ever, uncovering the etiology often requires complex endocrine testing. The human body has an amazing ability to tightly control blood glucose between 60 mg/dL and 150 mg/dL, despite times of large caloric intake (meals and snacks) and fasting (sleep). An intricate hormonal system governed by insulin and regulated by counter regulatory hormones such as growth hormone, cortisol, catecholamines, and glucagon allows for this constant precise control which is essential for the brain, given its minimal glycogen stores.
Hypoglycemia is generally defined as a serum glucose level <50 mg/dL. However, there is a wide range of serum glucose levels at which symptoms develop. Although hypoglycemic symptoms vary widely, Whipple’s triad reminds clinicians of the framework for making a diagnosis of hypoglycemia and includes:
- hypoglycemic symptoms
- a low serum blood glucose level documented while symptomatic
- reversal of the symptoms with glucose administration.
Symptoms of hypoglycemia fall into two categories: neuroglycopenic and autonomic.
Neuroglycopenic symptoms result directly from glucose deprivation in the brain and include confusion, fatigue, loss of consciousness, and seizures.
Autonomic responses result from norepinephrine released from postsynaptic ganglion and epinephrine re- leased from the adrenal medulla. Autonomic symptoms include sweating, hunger, tremor, anxiety, paresthesias, and palpitations.
The physical examination in the hypoglycemic patient is significant for pallor and diaphoresis. The heart rate and blood pressure may be elevated; however, this is not a universal find- ing. Focal neurological signs are possible, especially in elderly patients, and may mimic an acute cerebral event.
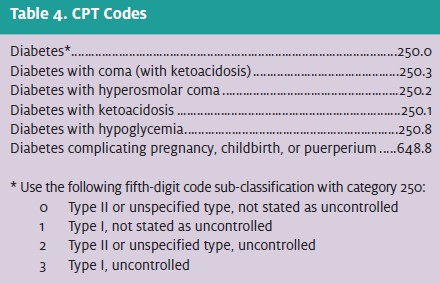
The etiology of hypoglycemia is broad and is best considered in three categories:
- Diabetes-related hypoglycemia is the most common etiology and results from excessive insulin administration either by error or during times of decreased insulin requirements (e.g., illness, weight loss).
- Reactive hypoglycemia may be seen in children with uncommon enzymatic defects and adults following gastric bypass surgery. The diagnosis of idiopathic postprandial hypoglycemia (functional hypoglycemia) is more difficult and controversial among the endocrine community, as serum glucose values fall below 50 mg/dL in more than 5% of healthy adults.
- Fasting hypoglycemia may result from medications, endocrine conditions, and severe illness. Culprit medications include those related to diabetes treatment, including insulin and sulfonylureas, as well as metformin and thiazolidinediones (if the latter two are combined with other diabetic medications).
Other medications that may result in hypoglycemia in- clude pentamidine, quinine, salicylates, and propranolol. Ethanol-related hypoglycemia results from ethanol’s inhibition of hepatic gluconeogenesis. Commonly, an ethanol drinking binge will result in glycogen depletion in chronic alcoholics who do not have the subsequent ability to complete gluconeogenesis while inebriated. Ethanol-related hypoglycemia is among the most dangerous etiologies, with mortality reports as high as 10%.
A thorough differential of hypoglycemia is provided in Table 2.
Initial evaluation of a patient with hypoglycemia should include testing Whipple’s triad (i.e., does the patient have hypoglycemic symptoms during a time when the serum glucose is documented to be low, and do they resolve with glucose administration?). Artifactually low glucose levels—a result of ongoing glucose metabolism after the sample is drawn or elevated blood counts as seen in leukemia—should be excluded. If a low serum glucose is confirmed, other lab values should be collected, including insulin, C peptide, sulfonylurea levels, cortisol, and ethanol levels.
Treatment
Urgent therapy for hypoglycemia involves the immediate administration of glucose or glucagon.
If the patient is mentally alert, glucose tablets or glucose-rich food such as rice, bread, fruits, or honey may be administered.
If the patient is obtunded, intravenous glucose (25 given in a 50% solution) should be administered as a bolus followed by a constant infusion of 5% or 10% dextrose.
Glucagon is a reasonable alternative when IV access is not possible, as glucagon can be administered either SQ or IM. Special consideration must be given to subjects with decreased glycogen pools (e.g., starvation patients, alcoholics, anorexic patients), as glucagon acts to stimulate glycogenolysis, and these subjects lack the necessary sustrate. Additional monitoring is required for these patients prior to discharge, as hypoglycemia may recur.
Preventing further hypoglycemic episodes requires an understanding of the etiology. Offending medications should be eliminated, and long-acting insulin analogues and sulfonylureas may result in prolonged hypoglycemia. Reactive hypoglycemia may respond to smaller, more frequent meals. Excessive endogenous insulin production re- quires detailed hormonal examination by an endocrinologist during a 72-hour fast.
A detailed position paper on hypoglycemia diagnosis and treatment was recently released by the American Diabetes Association,10 and an excellent review of the differential and evaluation is provided by F.J. Service.11
Summary
As diabetes continues to become more prevalent in the U.S., glycemic emergencies may be encountered with increasing frequency in the urgent care setting. Prompt management and treatment is needed to stabilize these patients prior to transfer to the hospital.
REFERENCES
- Diabetes Care. 2008;31:1-20.
- Service Hypoglycemic disorders. New Engl J Med. 1995;332:1144-1152.
- Basu A, Close CF, Jenkins D, et Persisting mortality in diabetic ketoacidosis. Di- abet Med. 1992;10:282-289.
- Malone ML, Gennis V, Goodwin Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc. 1992;40:1100-1104.
- Vermeersch N, Stolte C, Fostier K, et An unusual case of hyperglycemia, abdom- inal pain, and increased anion gap acidosis. J Emerg Med. Epub, 2008.
- Morris LR, Murphy MB, Kitabchi Bicarbonate therapy in severe diabetic ketoaci- dosis. Ann Intern Med. 1986;105:836-840.
- Kitabchi AE, Umpierrez GE, Barrett EJ, et Management of hyperglycemic crisis in patients with diabetes. Diabetes Care. 2001;24:131-153.
- Kitabchi AE, Wall Diabetic ketoacidosis. Med Clin North Am. 1995;79:9-37.
- Kitabchi AE, Umpierrez GE, Fisher JN, et Thirty years of personal experience in hyperglycemic crisis: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endrinol Metab. Epub, 2008.
- American Diabetes Association: Defining and reporting hypoglycemia in diabetes: A report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes
Care. 2005;28:1245. - Service FJ. Hypoglycemic disorders. New Engl J Med. 1995;332:1144-1152.