Published on
Urgent Message: Patients commonly present with respiratory symptoms in the urgent care setting and not infrequently complain of some degree of shortness of breath—or dyspnea. It is critical for clinicians to have comfort with the clinical assessment and appropriate use of diagnostic resources for the dyspneic patient. Identifying patients requiring immediate emergency department referral is an important skill.
Evan Price, DO; Eric Patten, MD; Shakil Hossain, DO; Michael Weinstock, MD
Citation: Price E, Patten E, Hossain S, Weinstock M. Dyspnea in the Urgent Care: Differentiating Benign From ‘Can’t Miss.’ J Urgent Care Med. 2024; 18(9):13-21
Introduction
Dyspnea has a broad differential of worrisome diagnoses ranging from flash pulmonary edema and pneumothorax to more benign conditions such as viral upper respiratory infection (URI) and asthma. This article will cover the “can’t miss” diagnoses that may present to the urgent care (UC) center and review recommendations for the diagnosis of chronic obstructive pulmonary disease (COPD), asthma, and pneumonia. Patient descriptions of dyspnea in conjunction with the history, physical exam, and testing will often lead clinicians to a diagnosis within 1 of 5 distinct categories:
- Airway
- Pulmonary
- Cardiac
- Systemic
- Central
Descriptors of the Term ‘Dyspnea’
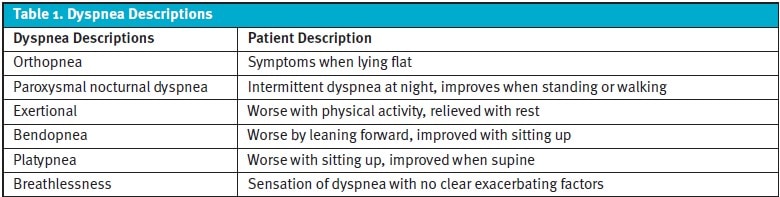
The descriptions and timing of a patient’s dyspnea are crucial in determining the most likely etiology and ruling out the more critical diagnoses. However, there is a significant variation in the understanding of much of this terminology, which may vary between the patient and the clinician.1 Wheezing, coughing, and fine crackles may point clinicians to a primary parenchymal or bronchial cause of dyspnea, such as asthma or COPD, but can also be caused by heart failure or a foreign body in the airway. The axiom that “all that wheezes is not asthma” applies to this reality. If a patient denies “shortness of breath” while sitting on the exam table in the UC, they may still answer affirmatively when asked the follow-up question regarding dyspnea with exertion or orthopnea.
History
Apart from general questions regarding onset and duration of symptoms, the history should probe for inciting triggers, alleviating factors, exposures, timing, onset, associated symptoms, descriptors of discomfort, and exposures (to smoking, chemicals, fumes, dust, etc.). Onset occurring within a few minutes to hours of presentation should raise considerations for acute coronary syndrome (ACS), anxiety, bronchospasm, pulmonary embolism (PE), pneumothorax (PTX), or foreign body aspiration. Dyspnea developing gradually over days to weeks can signify heart failure, anemia, or pneumonia. Malignancy causing anatomical obstruction of the respiratory tree may create progressive dyspnea which develops over months to years. Past diagnoses for causes of dyspnea (such as COPD or interstitial lung disease/pulmonary fibrosis), hospitalizations, response to treatments, and chart review of results of previous testing can also narrow the differential. Prematurely concluding, however, that a prior condition is responsible for the patient’s current presentation is an example of several common forms of cognitive biases, “premature closure” and “diagnosis momentum,” which can lead clinicians astray.2
Exam
- Breathing Patterns
In addition to a focused lung exam, observation of breathing patterns can suggest a variety of conditions.3 For example, clinicians may observe the ability of the patient to comfortably ambulate in the UC clinic and speak in full sentences. When anxiety is producing dyspnea, for instance, the patient may have normal or even-pressured speech and be restless or pacing in the exam room, whereas non-psychogenic causes often impair comfortable speech and movement. The use of the accessory muscles of respirations (indicated by retractions or nasal flaring) is ominous and may indicate impending respiratory failure. The Kussmaul respiratory pattern is characterized by fast, deep breaths that occur to compensate for severe metabolic acidosis as can be seen in diabetic ketoacidosis (DKA), sepsis, and renal failure.4 Cheyne-Stokes respirations, rare in UC, are characterized by cyclic episodes of rapid breathing followed by a period of apnea and can be seen in patients with coma, severe strokes, and end-stage heart failure.5,6 Prolonged expiratory phase suggests lower airway obstructive disease, like COPD or asthma.7,8 - Chest Wall And Neck
The chest wall should be examined for signs of trauma as well as the pattern of rise and fall. The neck should be examined for masses which may compress the airway or tracheal deviation. Patients with an upper airway obstruction, such as a foreign object or severe airway swelling due to croup, epiglottitis, or abscesses, may have stridor, drooling and may be sitting in a “tripod” position to maintain airway patency.9 - Lungs
Diffuse expiratory wheezing with decreased aeration suggests exacerbation of obstructive processes in patients with history of asthma, COPD, or chronic bronchitis, but can also be heard in heart failure.7,8 Unilateral decreased breath sounds may be present with a pneumothorax or pleural effusion.10 - Cardiovascular
The cardiovascular exam may reveal murmurs from valvular disease, gallop patterns of heart sounds suggestive of heart failure, or abnormal rate or rhythm, which may all cause a sense of dyspnea.11 - Abdominal
Abdominal exam may demonstrate liver enlargement or hepatojugular reflux which is suggestive of volume overload/heart failure. A distended abdomen from ascites, constipation, or bowel obstruction may cause dyspnea by limiting diaphragmatic excursion.11 - Extremities
Bilateral lower extremity edema can suggest heart failure, whereas unilateral extremity swelling in a patient with dyspnea should raise concern for deep vein thrombosis (DVT) and concomitant pulmonary embolism (PE).12
Testing
The testing most readily available in the UC setting includes chest radiography (CXR) and electrocardiogram (ECG) as well as pulse oximetry, point-of-care (POC) blood glucose, and possibly blood laboratory testing (either immediately available via POC testing or as rapid send-outs to an affiliated lab). The use of bedside ultrasonography (ie, point-of-care ultrasound [POCUS]) has also been shown to improve diagnostic accuracy in the evaluation of patients with undifferentiated dyspnea, if available.13
A. Chest Radiography
CXR is routinely available in UC and can demonstrate suggestive findings in cases of pneumonia, pneumothorax, pulmonary edema, heart failure, or malignancy. Chronic conditions, such as COPD, also have associated findings of hyperinflation such as flattened diaphragms, “dark” lungs from destruction of lung parenchyma, and enlarged retrosternal space (on lateral views).14
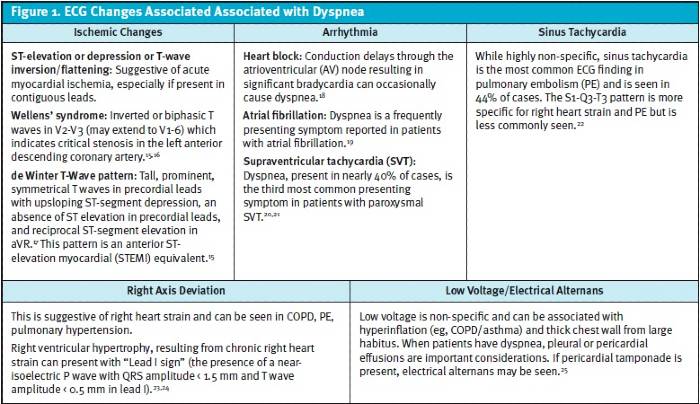
B. Electrocardiogram Many ECG findings can be suggestive of a concerning diagnosis, however, conversely, patients with dangerous etiologies for dyspnea may also have a normal ECG. Figure 1 reviews ECG findings to evaluate, which can offer clues to a patient’s dyspnea.
C. Finger-Stick Glucose Measurement
Dyspnea may be a presenting symptom in patients with DKA. Kussmaul respirations may be experienced as dyspnea when patients are compensating for metabolic acidosis. A capillary blood glucose is usually an appropriate screening test, although euglycemic DKA can occur, and it is important to note that in cases of high clinical suspicion, DKA must still be considered even without severe hyperglycemia.26
D. Pulse Oximetry
Pulse oximeters measure the oxygen concentration via detection of hemoglobin saturation within the blood. Most manufacturers claim accuracy within 3% of the actual value. Oxygen saturations below 88-90% define hypoxemia and suggest that patients require supplemental oxygen to maintain adequate oxygen delivery to tissues.27 Patients with new hypoxemia generally require hospital admission and benefit from emergent referral to the emergency department (ED). This is typically most safely accomplished by activating emergency medical services (EMS) as such patients require monitoring and supplemental oxygen while awaiting more definitive evaluation and treatment.
It is important to note that inaccurate readings are common with pulse oximeter devices, particularly if wave form monitoring is unavailable. Frequent causes of falsely abnormal fingertip pulse oximeter readings include poor perfusion/cold extremities, darker skin tones, and nail polish.28 Placing concerning oximetry readings within the patient’s overall clinical context and using alternate sites of measurement (eg, alternate hand, ear lobes, forehead) can minimize likelihood of inappropriate interpretation of false results.
E. Point-of-Care Ultrasound
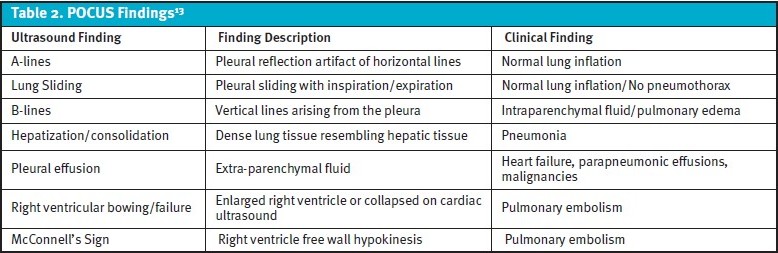
Although many UC centers currently do not have access to bedside ultrasound (ie, POCUS), it is frequently utilized in the inpatient and ED setting and can provide abundant information helpful for narrowing the differential diagnoses for undifferentiated dyspnea. POCUS has been shown to improve diagnostic accuracy when compared to standard clinical pathways alone in the assessment of dyspnea.29,30,31 Table 2 summarizes important POCUS findings related to the evaluation of dyspnea and their associated significance.
Airway
Dyspnea associated with drooling and/or stridor is suggestive of upper airway involvement. This can be identified with simple observation and does not require lung auscultation. Airway compromise is important to identify quickly to prevent delays in EMS activation. Airway obstruction may progress rapidly, making definitive airway management (eg, intubation or surgical airway options) more challenging for the emergency clinicians and risky for the patient.
Many different conditions can create obstruction in the upper airways. Important considerations for airway causes for UC clinicians to include in the differential diagnosis are as follows:
- Foreign body aspiration often occurs in toddlers and young children when placing objects such as small toys, coins, and beads in their mouths. Certain foods such as small nuts and berries are not recommended in this age range due to aspiration risks. Foreign body obstruction from aspiration is far less common in adults. Imaging with chest and neck radiographs can assist with localizing radiopaque foreign bodies, such as coins, but are less sensitive for identifying aspirated organic material.32
- Peritonsillar abscess (PTA) or retropharyngeal abscess (RPA) may cause upper airway compromise in severe cases. Oropharyngeal abscesses present more insidiously over days and typically are associated with fever, sore throat, dysphagia, trismus, muffled speech (“hot potato voice”), unilateral peritonsillar or posterior pharyngeal erythema, and swelling. RPA is predominantly a disease of childhood. In patients with PTA, significant uvula deviation is an ominous finding and portends greater risk of impending airway compromise.33
- Oropharyngeal aspiration commonly occurs secondary to dysphagia and should be suspected in patients with previous cerebral vascular accident, neuromuscular disorders (eg, myasthenia gravis), or dementia. Onset of dyspnea usually occurs seconds to minutes after aspiration. A CXR may demonstrate opacities consistent with pneumonitis. Antibiotics are not indicated for aspiration alone without signs of pneumonia (eg, fever, progressive cough, etc.).34
- Angioedema results from the leaking of fluid from the vasculature into an interstitial space and may be mediated by histamine or bradykinin.35 Angioedema is among the most common allergic disorders requiring hospitalization.36 Drug induced angioedema is most commonly related to ACE inhibitors (ACEi) and, to a lesser extent, angiotensin receptor blockers and can develop at any time during treatment but most commonly will occur within the first week of use.37 Affected patients present with external face, lips, mouth, throat, or extremities affected. Both hereditary angioedema and ACEi related angioedema can rapidly progress to airway compromise requiring intubation.38 The most appropriate treatment of angioedema is determined by the underlying cause. Prompt referral to the ED is recommended for severe or progressive cases where the airway is at risk of compromise.39 Imaging and labs are not required, and the diagnosis of angioedema is entirely based on characteristic exam findings.
- Epiglottitis historically was caused most commonly by Haemophilus influenzae (H. flu) in children, however, with the advent of vaccination against H. flu,the demographics of the illness have shifted towards adulthood. Patients with epiglottitis often present with fever, a muffled voice, low-pitched stridor, and dyspnea.32 Lateral neck radiographs may demonstrate inflammation of the epiglottis, including the classic “thumbprint” sign, but are insensitive and should not delay referral to the ED via EMS when epiglottitis is suspected. Diagnosis is confirmed via laryngoscopic visualization of the epiglottis in a controlled setting.40
- Croup is usually seen in young children during the fall and winter months related to viral URI and can present with dyspnea and stridor as well a “barky” or “seal-like” cough. Croup is diagnosed clinically, and treatment consists of systemic corticosteroids, typically dexamethasone, in all cases. Inhaled nebulized racemic epinephrine has been shown to have short term symptomatic benefits in moderate to severe croup (ie, patients with consistent stridor).41 Hot steam and humidified air have not been shown to offer significant benefit.42 However, the long-held wisdom of cold air relieving symptoms of croup has limited evidence to support its adjunctive role.42
- Vocal cord dysfunction can mimic upper airway disease. Symptoms include periodic noisy breathing, dyspnea, and cough. It is a diagnosis of exclusion and should be suspected when a patient has refractory symptoms despite appropriate treatments. Triggers include airborne irritants, physical exertion, psychologic conditions, rhinosinusitis, gastroesophageal reflux disease, and certain medications. Definitive diagnosis is via endoscopic evaluation.43
Pulmonary
Chronic obstructive lung diseases are a spectrum of disease states including asthma, chronic bronchitis, and emphysema. Patients with obstructive lung disease exacerbations present with end-expiratory wheezing, cough, and dyspnea.7
- Asthma is generally experienced in young adults and adolescents who have typically not been exposed to chronic parenchymal destruction and generally present with discrete flares related to environmental triggers, allergens, or respiratory infections. Asthma exacerbations are often more acute than COPD.44
- COPD is generally experienced in older patients with prolonged smoking history of greater than 30 years and presents with dyspnea, sputum production, and cough secondary to emphysema or chronic bronchitis. Onset is gradual with exacerbations linked to infectious etiologies, allergic, or idiopathic. Physical exam findings will show patients in mild-moderate distress, end-expiratory wheezing, and cough. Clinicians may see chronic changes of COPD on CXR such as hyperinflation.8 COPD is a frequent cause of UC visits and hospitalizations. Patients suffering from more severe disease are at a high risk of mortality and recurrent hospital admission.45 Treatments of obstructive lung disease flares are similar regardless of underlying etiology and include a combination of short-acting bronchodilators via inhaler or nebulizer and corticosteroid.46 Unlike the treatment of asthma exacerbation, antibiotics have been shown to decrease the severity and associated risks of COPD exacerbation.47
- Pneumonia is an infectious process of one or both lungs. Onset is typically gradual over several days. Pneumonia is a clinical diagnosis consisting of suggestive symptoms, such as productive cough and fever, and clinical signs, like tachypnea and abnormal breath sounds. A CXR, while not perfectly sensitive, may show variable types of infiltrates depending on the etiology. In addition to bacterial infections, fungal and viral etiologies as well as tuberculosis warrant consideration.48 The 2019 American Thoracic Society and Infectious Disease Society of America guidelines recommend antibiotic selection based on the likely pathogens. Patients without recent hospitalization or other risk factors for healthcare-associated pneumonia should be treated with antibiotics according to guidelines for community-acquired pneumonia. Antibiotic selection should also be driven by patients’ underlying co-morbidities.34
- PE is a cause of dyspnea that warrants consideration given its heterogenous manner of presentation and relatively high associated morbidity and mortality. Symptoms and signs suggestive of PE include tachycardia, tachypnea, cough, hemoptysis, and dyspnea. Risk calculators such as the Wells score and Pulmonary Embolism Rule Out Criteria (PERC) rule have been validated to risk stratify patients when PE is considered. If the patient is PERC-negative in UC, generally no further testing for PE is needed, however, caution should be advised in such cases if no alternate cause of dyspnea can be determined.49 D-dimer, which is rarely immediately available in the UC setting, can be used to exclude PE in low- and moderate-risk patients. In high-risk patients, computed tomography angiography of the pulmonary arteries is recommended to confirm or exclude the diagnosis.50
- PTX occurs when air becomes trapped in the pleural space. Classically, presentation is a sudden, sharp, ipsilateral, pleuritic pain with associated dyspnea. Patients are typically at rest during the occurrences, which may occur after blunt or penetrating chest trauma or spontaneously.10 Auscultation may reveal decreased or absent breath sounds. CXR is less sensitive than ultrasound or computed tomography for PTX but can demonstrate a lack of peripheral lung markings and a visible edge of the visceral pleura.51 When PTX is identified in UC, patients should be referred to an ED immediately, however, for a small PTX in a clinically stable patient, EMS activation may be unnecessary. Depending on the size and severity of symptoms, recent evidence has shown that small pneumothoraces (< 2 cm on CXR or < 32% of ipsilateral lung field on CXR) do not benefit from tube thoracostomy and can be monitored with serial radiography.52,53
Cardiac
Cardiac causes of dyspnea include congestive heart failure (CHF), acute coronary syndrome (ACS), pericarditis with pericardial effusion, cardiac valvular disease, and cardiac dysrhythmia.
- CHF and CHF exacerbations are common cardiac causes of dyspnea. Nearly all patients with CHF will report some degree of dyspnea. Other less universal CHF symptoms include orthopnea, paroxysmal nocturnal dyspnea, and peripheral swelling. Physical exam findings suggestive of CHF include extra heart sounds (ie, gallop), rales, jugular distension, and pitting edema of the lower extremities.54 CXR may show pulmonary edema and/or pleural effusions, which may contribute to dyspnea. In addition to clinical findings, an elevated B-natriuretic peptide laboratory value is suggestive of the diagnosis, which can be confirmed by echocardiography.55
- ACS can present with significant dyspnea. While ACS most commonly is associated with chest pain as well, it may occur without chest pain/discomfort in women, diabetics and the elderly.56 In patients who are dyspneic from a suspected cardiac etiology, rapid ECG is critical for risk stratification. Suggestive ECG findings for coronary occlusion/ischemia include ST-T wave abnormalities. Comparison with a prior ECG is highly valuable, when available.15 Figure 1 represents many ECG findings in patients who present with dyspnea.
- Pericarditis, pericardial effusion, and tamponade may all present with dyspnea. Pericarditis most commonly presents with sharp, pleuritic chest pain; improvement in the pain with leaning forward is also suggestive of the diagnosis. Pericarditis with effusion can occur after viral infections, but also can be related to autoimmune disease, renal failure, or malignancy.57 Dyspnea associated with pericarditis should raise concern for pericardial effusion and pericardial tamponade. Signs of pericardial tamponade include tachycardia, hypotension, jugular venous distension, and pulsus paradoxus. If tamponade is present, the ECG may demonstrate electrical alternans and a low QRS voltage, but a normal ECG does not exclude the diagnosis.57 While not widely available in UC, POCUS is particularly helpful for ruling out pericardial effusion. Cases of symptomatic pericardial effusion are treated with decompression via pericardiocentesis or pericardial window.58 Patients with pericarditis without significant effusion can be managed with non-steroidal anti-inflammatory drugs or colchicine as outpatients.59,60
- Valvular heart disease represents a broad category of disorders. Among the valvular disorders aortic stenosis (AS) and mitral regurgitation (MR) are most commonly associated with complaints of dyspnea. In AS, patients may demonstrate pulsus parvus et tardus, crescendo-decrescendo systolic murmur, and left ventricular hypertrophy on ECG. MR, on the other hand, causes a diastolic murmur. Valvular disorders are identified and scored in severity on echocardiogram. Both AS and MR have significant associated morbidity and mortality if untreated.61 In recent decades, the advent of intravascular approaches to valvular heart procedures have allowed for many more patients than previously to be considered candidates for valve repair or replacement.61
Systemic
Dyspnea may be experienced by patients as a result of systemic illness, especially those that result in disruption of pH, predominantly in cases of metabolic acidosis, and/or impairment of oxygen delivery to the tissues.62 Patients with these conditions will increase their respiratory rate in an effort to compensate for the underlying derangement.63
When tachypnea is identified, etiologies of metabolic acidosis should be considered including uremia (eg, renal failure), lactic acidosis (eg, sepsis and other forms of shock or salicylate poisoning), or ketoacidosis (eg, diabetes).64 Anemia is a well-established cause of dyspnea via the compromised ability of the blood to transport and deliver oxygen to the tissues.65,66 While the severity of anemia required to cause dyspnea is variable and without the existence of well-defined cut-offs, it has been demonstrated that the more severe the anemia the more likely a patient is to be dyspneic.67 Physical exam as a non-specific method by which anemia can be assessed with patients often appearing pale. Conjunctiva pallor is useful to assess for presence of anemia with one study showing it can often rule out significant anemia.67
Central
Acute central causes of dyspnea can be seen in psychogenic disorders such as anxiety, panic disorders, and post-traumatic stress disorder as well as in primary central-nervous system disorders.
Psychogenic disorders may present with dyspnea, though this can also occur with medical conditions as well.68 The diagnosis of these conditions is clinical, requiring a thorough history taking and physical examination as laboratory testing and imaging are non-diagnostic and reserved for ruling out alternative underlying conditions.69 Patient’s describing episodes of rapid breathing associated with specific behavioral triggers, such as certain social interactions, may be important clinical clues.70
High-altitude periodic breathing presents with dyspnea characterized by alternating periods of apnea and hypopnea followed by hyperventilation and is encountered more frequently at higher elevations and in patient’s completing more rapid ascents.71 This Cheyne-Stokes breathing pattern can also be seen in terminal/end-stage congestive heart failure.4,71
Conclusion
While the causes of dyspnea are broad, many patients who complain of shortness of breath can be adequately assessed and triaged in the UC setting. The systems-based framework of categorizing the differential for patients with dyspnea presented in this article offers a simple and easy-to-apply tool for UC practice. For many conditions, definitive treatment and/or reassurance in UC is appropriate after a thorough clinical evaluation and consideration of the broad list of differential diagnoses outlined herein. Despite the limited available diagnostic capabilities, many patients can be adequately risk stratified with clinical assessment and the POC diagnostic tools available in UC such that discharge home with appropriate follow-up and return precautions is safe and reasonable. Therefore, it is important for UC clinicians to develop comfort with systematically reviewing the differential diagnoses that may lead to complaints of shortness of breath to both avoid over-testing lower risk patients and misdiagnosing the rarer “can’t miss” presentations.
Manuscript submitted January 21, 2024; accepted May 6, 2024.
References
- von Leupoldt A, Balewski S, Petersen S, et al. Verbal descriptors of dyspnea in patients with COPD at different intensity levels of dyspnea. Chest. 2007;132(1):141-147. doi:10.1378/chest.07-0103 + Scano G, Stendardi L, Grazzini M. Understanding dyspnoea by its language. Eur Respir J. 2005;25(2):380-385. doi:10.1183/09031936.05.00059404)
- Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002 Nov;9(11):1184-204. doi: 10.1111/j.1553-2712.2002.tb01574.x. PMID: 12414468. (Accessed 12/10/23)
- Morgan W C, Hodge H L . 1998, February 15. Diagnostic evaluation of Dyspnea. Am Fam Physician. Accessed at https://www.aafp.org/pubs/afp/issues/1998/0215/p711.html#afp19980215p711-b2 (Accessed 1/5/24)
- Shahid W, Khan F, Makda A, Kumar V, Memon S, Rizwan A. Diabetic Ketoacidosis: Clinical Characteristics and Precipitating Factors. Cureus. 2020 Oct 04;12(10):e10792. (Accessed 12/10/23)
- Yamaoka-Tojo M. Is It Possible to Distinguish Patients with Terminal Stage of Heart Failure by Analyzing Their Breathing Patterns? Int Heart J. 2018;59(4):674-676. (Accessed 12/10/23)
- Kim Y, Kim S, Ryu DR, Lee SY, Im KB. Factors Associated with Cheyne-Stokes Respiration in Acute Ischemic Stroke. J Clin Neurol. 2018 Oct;14(4):542-548. (Accessed 12/11/23)
- Wise R. Chronic obstructive pulmonary disease (COPD) – pulmonary disorders. Merck Manual Professional Edition. https://www.merckmanuals.com/professional/pulmonary-disorders/chronic-obstructive-pulmonary-disease-and-related-disorders/chronic-obstructive-pulmonary-disease-copd
- Ruvuna L, Sood A. Epidemiology of Chronic Obstructive Pulmonary Disease. Clin Chest Med. 2020 Sep;41(3):315-327. doi: 10.1016/j.ccm.2020.05.002. PMID: 32800187.
- Hammer J. 2004. Acquired upper airway obstruction. Paediatric Respiratory Reviews, 5(1), 25-33.
- Hallifax RJ, Goldacre R, Landray MJ, Rahman NM, Goldacre MJ. Trends in the Incidence and Recurrence of Inpatient-Treated Spontaneous Pneumothorax, 1968-2016. JAMA. 2018 Oct 9;320(14):1471-1480. doi: 10.1001/jama.2018.14299. PMID: 30304427; PMCID: PMC6233798.
- Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85(5):483-500. doi:10.4065/mcp.2009.0706
- Kahn, S. R. (1998). The clinical diagnosis of deep venous thrombosis. Archives of Internal Medicine, 158(21), 2315. https://doi.org/10.1001/archinte.158.21.2315
- Qaseem A, Alguire P, Dallas P, et al. Appropriate Use of Point-of-Care Ultrasonography in Patients With Acute Dyspnea in Emergency Department or Inpatient Settings: A Clinical Guideline From the American College of Physicians. Ann Intern Med. Published online April 27, 2021. Accessed January 5, 2024. doi:10.7326/M20-7844.
- Cardinale L, Volpicelli G, Lamorte A, Martino J; Andrea Veltri. Revisiting signs, strengths and weaknesses of Standard Chest Radiography in patients of Acute Dyspnea in the Emergency Department. J Thorac Dis. 2012 Aug;4(4):398-407. doi: 10.3978/j.issn.2072-1439.2012.05.05. PMID: 22934143; PMCID: PMC3426742.
- Writing Committee, Kontos MC, de Lemos JA, et al. 2022 ACC Expert Consensus Decision Pathway on the Evaluation and Disposition of Acute Chest Pain in the Emergency Department: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(20):1925-1960. doi:10.1016/j.jacc.2022.08.750
- Coutinho Cruz M, Luiz I, Ferreira L, Cruz Ferreira R. Wellens’ Syndrome: A Bad Omen. Cardiology. 2017;137(2):100-103. doi:10.1159/000455911. Accessed December 28, 2023.
- Littmann L. (The Dressler – de Winter sign of acute proximal LAD occlusion. Journal of Electrocardiology. 2018;51(1), 138–139. https://doi.org/10.1016/j.jelectrocard.2017.08.024. Accessed 1/12/24.
- Williams S. A 62-year-old woman with dizziness and palpitations. J Urgent Care Med. Published August 29, 2018. Accessed March 22, 2024. Available from: https://www.jucm.com/62-year-old-woman-dizziness-palpitations/
- Paulus Kirchhof, Stefano Benussi, Dipak Kotecha, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. doi:10.1093/eurheartj/ehw210.
- Yetkin E, Ozturk S, Cuglan B, Turhan H. Clinical presentation of paroxysmal supraventricular tachycardia: evaluation of usual and unusual symptoms. Cardiovasc Endocrinol Metab. 2020 May 15;9(4):153-158. doi: 10.1097/XCE.0000000000000208. PMID: 33225230; PMCID: PMC7673777.
- Colucci RA, Silver MJ, Shubrook J. Common types of supraventricular tachycardia: Diagnosis and management. Am Fam Physician. Published online October 15, 2010. Accessed from https://www.aafp.org/pubs/afp/issues/2010/1015/p942.html.
- Ullman, E., Brady, W. J., Perron, A. D., Chan, T., Mattu, A. Electrocardiographic manifestations of pulmonary embolism. Am J Emergency Med. 2001;19(6), 514-519.
- Goudis CA, Konstantinidis AK, Ntalas IV, Korantzopoulos P. Electrocardiographic abnormalities and cardiac arrhythmias in chronic obstructive pulmonary disease. Int J Cardiol. 2015;199:264-273.
- Gupta P, Jain H, Gill M, Bharaj G, Khalid N, Chaudhry W, Chhabra L. Electrocardiographic changes in Emphysema. World J Cardiol. 2021 Oct 26;13(10):533-545. doi: 10.4330/wjc.v13.i10.533. PMID: 34754398; PMCID: PMC8554360.
- 25. Jesper K. Jensen, Steen Hvitfeldt Poulsen. Cardiac tamponade: A clinical challenge. European Society of Cardiology. https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15/Cardiac-tamponade-a-clinical-challenge. Accessed 3/23/24.
- Nasa P, Chaudhary S, Shrivastava PK, Singh A. Euglycemic diabetic ketoacidosis: A missed diagnosis. World J Diabetes. 2021 May 15;12(5):514-523. doi: 10.4239/wjd.v12.i5.514. PMID: 33995841; PMCID: PMC8107974.
- Chan, E. D., Chan, M. M., Chan, M. M. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respiratory Med. 2013; 107(6), 789–799. https://doi.org/10.1016/j.rmed.2013.02.004. Accessed 12/26/23.
- Silverston P, Ferrari M, Quaresima V. Pulse oximetry in primary care: factors affecting accuracy and interpretation. Br J Gen Pract. 2022 Feb 24;72(716):132-133. doi: 10.3399/bjgp22X718769. PMID: 35210248; PMCID: PMC8884444.
- Gerald Gartlehner, Gernot Wagner, Lisa Affengruber, et al. Point-of-Care Ultrasonography in Patients With Acute Dyspnea: An Evidence Report for a Clinical Practice Guideline by the American College of Physicians. Ann Intern Med.2021;174:967-976. Epub 27 April 2021. doi:10.7326/M20-5504. Accessed 3/23/24.
- Murali A, Prakash A, Dixit R, Juneja M, Kumar N. Lung ultrasound for evaluation of dyspnea: a pictorial review. Acute Crit Care. 2022 Nov;37(4):502-515. doi: 10.4266/acc.2022.00780. Epub 2022 Nov 21. PMID: 36480902; PMCID: PMC9732207. Accessed 3/23/24.
- 31. Sosland, Rachel P., and Kamal Gupta. “McConnell’s sign.” Circulation, vol. 118, no. 15, 7 Oct. 2008, https://doi.org/10.1161/circulationaha.107.746602. Accessed 3/23/24
- Pfleger A, Eber E. Management of acute severe upper airway obstruction in children. Paediatr Respir Rev. 2013 Jun;14(2):70-7. doi: 10.1016/j.prrv.2013.02.010. Epub 2013 Apr 16. PMID: 23598067. Accessed 12/26/23
- Jenkins IA, Saunders M. Infections of the airway. Paediatr Anaesth. 2009 Jul;19 Suppl 1(Suppl 1):118-30. doi: 10.1111/j.1460-9592.2009.02999.x. PMID: 19572851; PMCID: PMC7168042. Accessed 12/26/23
- Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, Griffin MR, Metersky ML, Musher DM, Restrepo MI, Whitney CG. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67. doi: 10.1164/rccm.201908-1581ST. PMID: 31573350; PMCID: PMC6812437.
- Depetri F, Tedeschi A, Cugno M. Angioedema and emergency medicine: From pathophysiology to diagnosis and treatment. Eur J Intern Med. 2019 Jan;59:8-13. doi: 10.1016/j.ejim.2018.09.004. Epub 2018 Sep 13. PMID: 30220453. Accessed 1/9/24
- Lin RY, Cannon AG, Teitel AD. Pattern of hospitalizations for angioedema in New York between 1990 and 2003. Ann Allergy Asthma Immunol. 2005 Aug;95(2):159-66. doi: 10.1016/S1081-1206(10)61206-9. PMID: 16136766. Accessed 1/9/24
- Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: A review of the literature. J Clin Hypertens (Greenwich). 2017 Dec;19(12):1377-1382. doi: 10.1111/jch.13097. Epub 2017 Oct 10. PMID: 28994183; PMCID: PMC8031276. Accessed 1/8/24
- Kazandjieva J, Christoff G. Angioedema as a systemic disease. Clin Dermatol. 2019;37(6):636-643. doi:10.1016/j.clindermatol.2019.07.035 Accessed 12/27/23
- Macy E. Practical Management of New-Onset Urticaria and Angioedema Presenting in Primary Care, Urgent Care, and the Emergency Department. Perm J. 2021 Nov 22;25:21.058. doi: 10.7812/TPP/21.058. PMID: 35348101; PMCID: PMC8784078.
- Isakson M, Hugosson S. Acute epiglottitis: epidemiology and Streptococcus pneumoniae serotype distribution in adults. J Laryngol Otol. 2011;125(4):390-393. doi:10.1017/S0022215110002446
- Johnson DW. Croup. BMJ Clin Evid. 2014 Sep 29;2014:0321. PMID: 25263284; PMCID: PMC4178284. Accessed 12/28/23
- Siebert JN, Salomon C, Taddeo I, Gervaix A, Combescure C, Lacroix L. Outdoor Cold Air Versus Room Temperature Exposure for Croup Symptoms: A Randomized Controlled Trial. Pediatrics. 2023;152(3):e2023061365. doi:10.1542/peds.2023-061365
- Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. Published online January 15, 2010. Accessed from https://www.aafp.org/pubs/afp/issues/2010/0115/p156.html. Accessed January 6, 2024.
- Dharmage SC, Perret JL, Custovic A. Epidemiology of Asthma in Children and Adults. Front Pediatr. 2019 Jun 18;7:246. doi: 10.3389/fped.2019.00246. PMID: 31275909; PMCID: PMC6591438.
- Lindenauer PK, Dharmarajan K, Qin L, Lin Z, Gershon AS, Krumholz HM. Risk Trajectories of Readmission and Death in the First Year after Hospitalization for Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2018 Apr 15;197(8):1009-1017. doi: 10.1164/rccm.201709-1852OC. PMID: 29206052; PMCID: PMC5909167.
- Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs Conventional Glucocorticoid Therapy in Acute Exacerbations of Chronic Obstructive Pulmonary Disease: The REDUCE Randomized Clinical Trial. JAMA. 2013;309(21):2223–2231. doi:10.1001/jama.2013.5023
- Siddiqi A, Sethi S. Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008;3(1):31-44. doi: 10.2147/copd.s1089. PMID: 18488427; PMCID: PMC2528209.
- Htun TP, Sun Y, Chua HL, Pang J. Clinical features for diagnosis of pneumonia among adults in primary care setting: A systematic and meta-review. Sci Rep. 2019 May 20;9(1):7600. doi: 10.1038/s41598-019-44145-y. PMID: 31110214; PMCID: PMC6527561.
- Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi:10.1111/j.1538-7836.2008.02944.x
- Freund Y, Cohen-Aubart F, Bloom B. Acute Pulmonary Embolism: A Review. JAMA. 2022 Oct 4;328(13):1336-1345. doi: 10.1001/jama.2022.16815. PMID: 36194215.
- Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev. 2010 Sep;19(117):217-9. doi: 10.1183/09059180.00005310. PMID: 20956196; PMCID: PMC9487279.
- Brown SGA, et al. Conservative versus Interventional Treatment for Spontaneous Pneumothorax. N Engl J Med. 2020 Jan 30;382(5):405-415
- MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010 Aug;65 Suppl 2:ii18-31. doi: 10.1136/thx.2010.136986. PMID: 20696690.
- Jessup, M., & Brozena, S. (2003). Heart failure. New England Journal of Medicine, 348(20), 2007–2018. https://doi.org/10.1056/nejmra021498
- Inamdar AA, Inamdar AC. Heart Failure: Diagnosis, Management and Utilization. J Clin Med. 2016 Jun 29;5(7):62. doi: 10.3390/jcm5070062. PMID: 27367736; PMCID: PMC4961993.
- Brieger D, Eagle KA, Goodman SG, Steg PG, Budaj A, White K, Montalescot G; GRACE Investigators. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004 Aug;126(2):461-9. doi: 10.1378/chest.126.2.461. PMID: 15302732.
- Imazio, M., Gaita, F., LeWinter, M. Evaluation and treatment of pericarditis. JAMA. 2015; 314(14), 1498. https://doi.org/10.1001/jama.2015.12763
- Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA. 2007 Apr 25;297(16):1810-8. doi: 10.1001/jama.297.16.1810. PMID: 17456823.
- Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964. doi:10.1093/eurheartj/ehv318
- Ismail TF. Acute pericarditis: Update on diagnosis and management. Clin Med (Lond). 2020 Jan;20(1):48-51. doi: 10.7861/clinmed.cme.20.1.4. PMID: 31941732; PMCID: PMC6964178.
- Pittman RN. Regulation of Tissue Oxygenation. San Rafael (CA): Morgan & Claypool Life Sciences; 2011. Chapter 4, Oxygen Transport. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54103/
- Fenves AZ, Emmett M. Approach to Patients With High Anion Gap Metabolic Acidosis: Core Curriculum 2021. Am J Kidney Dis. 2021;78(4):590-600. doi:10.1053/j.ajkd.2021.02.341
- Lactic acidosis. (2015). New England Journal of Medicine, 372(11), 1076–1079. https://doi.org/10.1056/nejmc1500327
- Boutou AK, Stanopoulos I, Pitsiou GG, Kontakiotis T, Kyriazis G, Sichletidis L, Argyropoulou P. Anemia of chronic disease in chronic obstructive pulmonary disease: a case-control study of cardiopulmonary exercise responses. Respiration. 2011;82(3):237-45. doi: 10.1159/000326899. Epub 2011 May 11. Erratum in: Respiration. 2011;82(6):521. PMID: 21576921.
- Weckmann G, Kiel S, Chenot JF, Angelow A. Association of Anemia with Clinical Symptoms Commonly Attributed to Anemia-Analysis of Two Population-Based Cohorts. J Clin Med. 2023 Jan 24;12(3):921. doi: 10.3390/jcm12030921. PMID: 36769569; PMCID: PMC9918126.
- Cote C, Zilberberg MD, Mody SH, Dordelly LJ, Celli B. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J. 2007 May;29(5):923-9. doi: 10.1183/09031936.00137106. Epub 2007 Jan 24. PMID: 17251227.
- Kalantri A, Karambelkar M, Joshi R, Kalantri S, Jajoo U. Accuracy and reliability of pallor for detecting anaemia: a hospital-based diagnostic accuracy study. PLoS One. 2010;5(1):e8545. Published 2010 Jan 1. doi:10.1371/journal.pone.0008545
- American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, (DSM-5). 2013.
- Kyriakoulis P, Kyrios M. Biological and cognitive theories explaining panic disorder: A narrative review. Front Psychiatry. 2023 Jan 30;14:957515. doi: 10.3389/fpsyt.2023.957515. PMID: 36793941; PMCID: PMC9924294.
- Narat Srivali, Lauren Tobias, 1037 Central sleep apnea due to high altitude periodic breathing, Sleep, Volume 46, Issue Supplement. May 2023, Page A455, https://doi.org/10.1093/sleep/zsad077.1037
- Andreas S, Hagenah G, Möller C, Werner GS, Kreuzer H. Cheyne-Stokes respiration and prognosis in congestive heart failure. Am J Cardiol. 1996;78(11):1260-1264.
Evan Price, DO; Eric Patten, MD; Shakil Hossain, DO; Michael Weinstock, MD

