Published on
Urgent message: Increasingly, patients with symptoms of acute infection run to their nearest urgent care center. As such, urgent care clinicians can contribute greatly to national efforts to save lives by stemming the growth of antibiotic resistance through good antibiotic stewardship.
Introduction
Despite being a recent healthcare phenomenon, urgent care centers are responsible for a growing percentage of outpatient healthcare, with an estimated 160 million total annual visits at more than 9,300 sites in the United States.1,2 This is attributed to long wait times in hospital emergency departments for nonemergent care, the shortage of primary care providers, a greater number of patients entering the healthcare system due to the Affordable Care Act, and patients’ desire for immediate access to care.2 These clinics do not require appointments, are usually conveniently located, are open 7 days a week, and have extended evening hours. This makes them an attractive option for patients seeking medical care for common episodic illnesses.
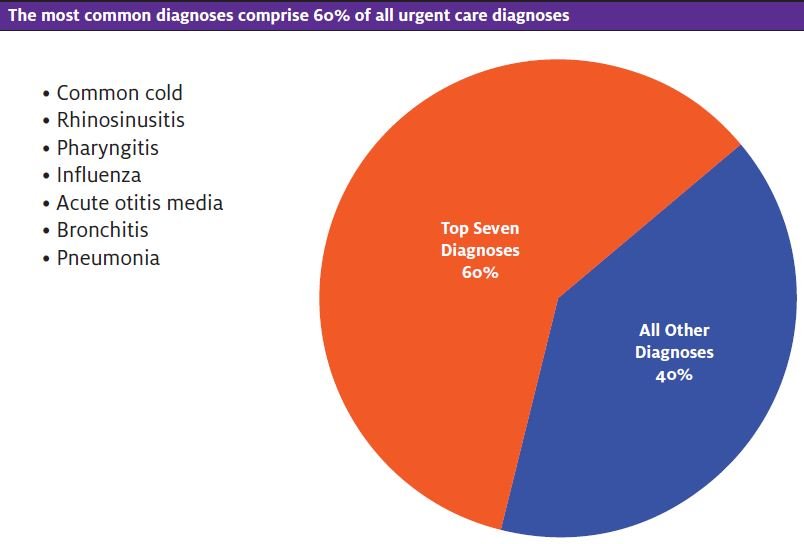
Urgent care is considered a cross between emergency care and family practice, with the predominant focus on acute presentations of disorders on the lower end of disease severity.3 The majority of patients present with symptoms of acute respiratory infections such as cough, fever, and sore throat and are diagnosed with the common cold, rhinosinusitis, pharyngitis, influenza, acute otitis media, bronchitis, and pneumonia. These account for up to 60% of patient visits.1,4
While these problems can be viral or bacterial in nature, they are often associated with antibiotic use. It is estimated that up to 50% of antibiotics prescribed in outpatient settings are either unnecessary or are inappropriate in selection, dose, or duration.5 This type of prescribing is the main factor contributing to antibiotic resistance, making this a concern in urgent care sites and requiring the education of providers.5-7
Antibiotic Resistance
Antibiotic resistance is a serious public health threat in the U.S., resulting in more than 2 million people who become ill with drug-resistant organisms annually; 23,000 die as a result.7 While antibiotic resistance was once only associated with hospital settings, recent data show there are now resistant organisms in outpatient settings.8 Clinicians play a part by giving antibiotics for illnesses when they are not needed. Some reasons cited for this are patient expectations, limited time with patients, poor communication, and the uncertainty of a diagnosis.9
A poll of urgent care providers found that 17% of respondents reported writing a prescription for an antibiotic even when a viral infection was suspected because patients demanded one.10 Since providers in urgent care usually do not have an established relationship with patients, it may be more difficult to resist the pressure when patients request an antibiotic. Diagnosis uncertainly also influences when antibiotics are prescribed. Many URIs initially present with similar symptoms, so it can be difficult to distinguish problems where an antibiotic truly is needed. Physical exam findings such as fever, purulent sputum, and tonsillar exudate have been found to have higher rates of antibiotic prescriptions for URIs.11 A national plan for antibiotic stewardship in outpatient settings has been developed to combat antibiotic resistance. This plan incorporates the use of clinical guidelines to assist providers in making an appropriate diagnosis and treatment for many of the common disorders in urgent care.12
In order to understand the problem of inappropriate antibiotics in urgent care, it is important to learn about common clinical problems and how antibiotics are used for those conditions. Typical upper respiratory conditions seen are among those associated with inappropriate prescribing.5 It is well known that the majority of URIs, including rhinosinusitis, acute bronchitis, the common cold, and pharyngitis are viral and will not respond to antibiotics.5 It is important to distinguish a viral from bacterial causes using established criteria and current evidence to support clinical decision making.
Common Conditions in Urgent Care
The Common Cold
A cold is a self-limiting, nonspecific upper respiratory infection that is the most frequent acute illness in the U.S. Children have up to seven episodes per year, and adults have two to three colds annually.13 There are more than 200 viruses that cause the common cold, and these vary by season. Symptoms often include a low-grade fever, cough, rhinorrhea, nasal congestion, sore throat, headache, and body aches. These vary in severity, are usually not severe, and last up to 10 days. Symptoms may last longer in patients who smoke. The physical exam findings in most patients will show few abnormalities, and the lower respiratory tract is not usually affected. Lung examination is typically clear.13,14
The common cold should be differentiated from other URIs, since antibiotics are never warranted. Patients with the common cold will have nasal discharge (usually clear), compared with those with acute bacterial rhinosinusitis (ABRS) who have purulent discharge and associated facial pressure. Patients with a cold may have a low-grade fever in the first few days, while severe illness in ABRS involves a fever >102.2⁰F. Patients should be monitored for complications of a common cold that may require antibiotics, including acute otitis media and ABRS.13-15
Anecdotally, some clinicians find it best to avoid using the term common cold with patients, in favor of upper respiratory infection, as the latter term “sounds” more like an illness than something “common.”

Pharyngitis
Pharyngitis, or “sore throat,” is also a common illness that may be viral or bacterial. It is estimated that 5%‒15% of sore throats in adults and 20%‒-30% in children are caused by group A beta-hemolytic Streptococcus (GAS), yet up to 70% of all patients diagnosed with pharyngitis will receive an antibiotic.5,16,17
Infection with GAS is the only common indication for antibiotics in patients with a sore throat, given to prevent complications. It may be difficult to distinguish GAS from viral pharyngitis, since they present with a sore throat and pain with swallowing. Fever, headache, or malaise may also be present; some patients also complain of “swollen glands” or anterior neck pain with enlarged lymph nodes. Children may also have abdominal pain or vomiting.18,19
The Centor or modified Centor criteria can be used to identify patients who are more likely to have GAS. Criteria include the presence of fever (>100.4⁰ F), tonsillar exudates, tender cervical lymphadenopathy, and absence of cough; some clinicians also consider the age of the patient.18,20 Patients who meet ≥2 Centor criteria should receive a rapid antigen detection test (RADT) to establish the diagnosis. A throat culture is recommended to confirm the results of the RADT in children, but not in adults due to the low incidence of GAS in the adult population.18,19 Antibiotics should be used only for those with a positive RADT test. A 10-day course of amoxicillin or penicillin V remains first-line therapy. Alternatives for penicillin-allergic patients include cephalexin, cefadroxil, clindamycin, or macrolides. It is important to note that there is an increased resistance of GAS to azithromycin and clindamycin.18,19
Rhinosinusitis
Acute rhinosinusitis is common in urgent care and associated with the most antibiotic prescriptions.5 Approximately 12% of adults in the U.S. were diagnosed with rhinosinusitis in 2012.21 It is estimated that 90%–98% of all cases of rhinosinusitis are viral,15 yet up to 50% of adults and 89% of children receive an antibiotic.5,22 Establishing an accurate diagnosis of ABRS is important in preventing antibiotic resistance. Clinical guidelines can assist the provider in identifying those patients who need an antibiotic.15,23,24
Presence of a purulent drainage with nasal obstruction and facial pain/pressure/fullness, or both, that persist without improvement for ≥10 days, or severe symptoms at the beginning of the illness in adults, are highly correlated with a bacterial infection.15,25 Severe symptoms include fever >102°F and purulent nasal discharge or facial pain lasting ≥3-4 days. Other symptoms indicating ABRS are fever, cough, fatigue, decreased or absent sense of smell, maxillary dental pain, and ear fullness or pressure.15 Patients may start with a viral URI and develop ABRS, indicated by URI symptoms for 5-6 days with some initial improvement, followed by worsening symptoms such as new-onset fever, headache, and change in nasal discharge.15,23,25
In the pediatric patient, the diagnosis of ABRS is based on the presence of a daytime cough, persistent illness with nasal discharge, or both for >10 days without any improvement. ABRS is also assumed if there is a severe onset of symptoms with a fever of at least 102.2⁰ F with purulent nasal discharge for 3 consecutive days.24
The initial treatment of uncomplicated acute rhinosinusitis in adults is watchful waiting and symptomatic relief.15,23 Pediatric patients with mild illness should also be offered observation for 3 days.15,24 Patients may need an antibiotic if they do not improve within 7 days or if they worsen at any time.15,23,24 One strategy to enhance compliance for observation is to give patients a postdated prescription along with instructions on when the antibiotic is needed. When antibiotics are warranted, there are conflicting recommendations about which antibiotic are first line for children and adults. Standard-dose amoxicillin/clavulanate is recommended for both children and adults by the Infectious Disease Society of America (ISDA),15 while amoxicillin may be considered appropriate for some pediatric patients.23 High-dose amoxicillin/clavulanate is considered for children and adults in regions with high levels of antibiotic resistance to strep pneumoniae, severe infection, children in daycare, antibiotic use or hospitalization within the previous month, presence of a comorbid condition, or extremes of age.15
Selection of the antibiotic should be based on the organism most likely causing the infection. There is an increased presence of B-lactamase-producing pathogens as a cause of ABRS in children, warranting the recommendation for amoxicillin/clavulanate.24 Duration of treatment is generally 5-7 days for adults and 10-14 days for children, based on severity of the illness or failure to respond within 5 days of treatment. Alternatives for adults are doxycycline, or a respiratory fluoroquinolone for those with penicillin allergies.15,23,24 Macrolides and trimethoprim-sulfamethoxazole are not recommended due to high levels of resistance against strep pneumoniae.15,23
Acute Bronchitis
Cough is a common presenting symptom in urgent care, and acute bronchitis (AB) is the most common diagnosis given to adults presenting with a cough in outpatient settings. It is defined as an acute respiratory infection that involves inflammation of the bronchi, manifesting as a cough, with or without sputum production and lasting ≤3weeks26,27 AB is most often is due to a virus, yet there are reports that up to 70% of patients diagnosed are given antibiotics.5 Initially, it may be difficult to distinguish the cough of AB from influenza, rhinovirus, coronavirus, and respiratory syncytial virus since cough is an early symptom. AB should be considered when a cough lasts >5 days, though it may last ≤3 weeks. The cough is associated with sputum production in 50% of patients, though the color of sputum is not indicative of bacterial infection.28 For a diagnosis of AB, there should be no evidence of pneumonia, acute asthma, or exacerbation of COPD.28,29
The primary evaluation should focus on identifying the correct diagnosis and any other possible causes of a cough that would need additional evaluation or treatment. Patients with AB usually have few systemic symptoms. They may have wheezing or chest wall tenderness from coughing. Physical exam findings consistent with AB are rhonchi (which often clear with coughing); wheezing may also be present.27-29 Indicators that suggest pneumonia or influenza are a combination of fever, cough, sputum production, and constitutional symptoms such as fatigue, malaise, and shaking chills. A chest x-ray may be required if pneumonia is suspected or in patients with abnormal vital signs (pulse >100/minute, respiratory rate >24 breaths/minute, or temperature >38°C), abnormal lung findings, or in patients of advanced age. Treatment of AB is symptomatic. Antibiotics are not recommended.26-28
Otitis Media
Acute otitis media (AOM) is an inflammation or infection of the middle ear that can occur at any age, though is most frequent in the pediatric population. It is the most common diagnosis for children’s outpatient sick visits and the most common reason for antibiotic use in pediatric patients, >80% of children receiving an antibiotic.5 AOM, or suppurative otitis media, is distinguished from otitis media with effusion (OME) by the presence of purulent fluid in the middle ear. OME is defined by the presence of fluid in the middle ear, without acute signs of illness or inflammation. OME may predispose a patient to AOM or occur as a result of AOM.30,31
There is no gold standard for the diagnosis of AOM.32 The history findings may differ by age. Symptoms in young are nonspecific and may include fever, irritability, sleep disturbances, poor feeding, nausea, and diarrhea. Older children more commonly present with fever and ear pain. Adults have ear pain and decreased hearing, often following a URI or allergic rhinitis. Purulent otorrhea may be present if the tympanic membrane (TM) has ruptured. The physical exam will show varying degrees of inflammation, bulging, or a perforated TM. Pneumatic otoscopy is also recommended to evaluate TM mobility and make visualization of a middle ear effusion more apparent. The diagnosis can be made in children if there is moderate-to-severe bulging of the TM or the presence of a new onset of otorrhea that is not due to otitis externa. Other criteria used for a diagnosis of AOM include mild bulging of the TM with new onset ear pain or significant erythema of the TM. The presence of a TM with bulging, impaired mobility with redness or cloudiness are strong predictors of AOM.31,32
Treatment is based on age and severity of illness. Observation in some pediatric patients is recommended. Clinicians should offer observation as an option for the initial treatment for children ≥6 months, with unilateral nonsevere AOM and unilateral or bilateral AOM in children aged ≥24 months without severe signs or symptoms. Antibiotics are started if there is worsening or failure to improve within 48–72 hours. It is critical to provide information on pain relief for these patients. Antibiotics are recommended for adults, all children 6 months‒23 months of age with mild bilateral disease, and for children ≥6 years who have severe unilateral or bilateral disease. Severe disease is described as moderate or severe ear pain for at least 48 hours, or a temperature of 102.2⁰F.31-33
Amoxicillin or amoxicillin/clavulanate for 10 days is first line for children and adults. Selection and dose are based on age, severity of disease for adults, and history of antibiotic use within the previous 30 days or the presence of purulent conjunctivitis in children. Alternative antibiotics include cefdinir, cefuroxime, cefpodoxime or ceftriaxone. It should be noted that macrolides have limited effectiveness against H influenza and S pneumoniae. 31-33
Solutions
The focus on combatting antibiotic resistance has extended to outpatient settings, including urgent care.34,35 The White House National Action Plan for Combating Antibiotic-Resistant Bacteria was developed in 2015 with a specific goal to reduce inappropriate antibiotic use in outpatient settings by 50% by 2020.35 Resources for providers, clinic personnel, and patients are available from The Centers for Disease Control (CDC),12,36-38 the IDSA, 39 and the Society for Healthcare Epidemiology of America.39 Recommended strategies include antibiotic stewardship practices, patient and provider education, and tracking and reporting improvements.
Antibiotic stewardship is the effort to measure and improve how antibiotics are prescribed by providers and then used by patients. Key components are for providers to align prescribing practices with evidence-based practice guidelines, evaluate their current practice, and learn to communicate effectively to patients when antibiotics are not needed.12,37 While providers are aware that antibiotics should only be prescribed when a bacterial infection is known or suspected, it appears that this is more difficult to implement given the inability to differentiate among some disorders in the early stages of illness. The use of guidelines can aid in decision making, address variations in practice, and assist in the education of providers and patients.40 Many organizations publish guidelines, so providers need to identify high-quality, trustworthy guidelines and use them to aid in making decisions about when antibiotics are needed.40 Studies show that the use of evidence-based practice guidelines to determine treatment, requiring providers to document/justify antibiotic use, and using treatment algorithms in electronic medical records to support appropriate antibiotic use have been effective at reducing antibiotic use.39,41-43
It may be difficult for providers to implement some of the newer recommendations such as watchful waiting or delayed antibiotics use. In addition, the desire to maintain positive satisfaction scores with patients who request an antibiotic, even when one is not warranted, can be powerful. Some patients feel they’ve received inadequate care if they leave the visit with “nothing.” In order to keep them happy without prescribing an antibiotic inappropriately, it may be helpful to counsel the patient respectfully by saying something along the lines of, “It’s not appropriate to give you antibiotics for this infection today, but what we can do…,” then offer other, more appropriate remedies (eg, cough medicine). It may also be helpful to have a printed sheet with recommendations for nonprescription medications.
In these situations, it is important to involve the patient or family by providing education about changes in recommendations, the appropriate use of antibiotics, the recommended treatment plan, and symptom management. Information about the potential harm of antibiotics, including adverse drug events, should be given.37,39 All urgent care staff can participate in antibiotic stewardship by communicating information to patients about the proper use of antibiotics. Providing written educational materials in waiting areas and patient rooms is one recommended approach. Urgent care sites should also perform quality improvement programs to identify improvements in practice. The CDC’s Get Smart program provides additional resources for patients and urgent care staff.37
Summary
As more patients receive care for common viral illnesses associated with inappropriate antibiotic use in urgent care sites, providers are being called upon to review their own prescribing patterns and develop strategies of antibiotic stewardship. Providers need to incorporate evidence-based practice recommendations to assist in the accurate diagnosis and treatment of commonly presenting illnesses and to differentiate viral from bacterial disease. Urgent care site providers and staff should use available resources and educational materials for patients and all staff related to antibiotic use, and monitor the effects of any programs developed.
Patricia Sweeney, PhD, CRNP, FNP-BC
Citation: Sweeney P. Improving appropriate antibiotic use for common clinical conditions in urgent care. J Urgent Care Med. June 2017. Available at: https://www.jucm.com/improving-appropriate-antibiotic-use-common-clinical-conditions-urgent-care/
References
- Urgent Care Association. 2012 Urgent Care Benchmarking Survey Results. Available at: www.ucaoa.org. Accessed November 19, 2016.
- American Academy of Urgent Care Medicine. Future of urgent care. Available at: http://aaucm.org/about/future/default.aspx. Accessed January 4, 2017.
- American Academy of Urgent Care Medicine. What is urgent care? Available at: https://aaucm.org/about/urgentcare/default.aspx. Accessed November 15, 2016.
- RAND Corporation. The evolving role of retail clinics. Available at: www.rand.org/pubs/research_briefs/RB9491-2.html. Accessed November 15, 2016.
- Fleming-Dutra K, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among U.S. ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873.
- Centers for Disease Control and Prevention. Measuring outpatient antibiotic prescribing. Get smart: know when antibiotics work. Available at: www.cdc.gov/getsmart/community/programs-measurement/measuring-antibiotic-prescribing.html. Accessed January 4, 2017.
- Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance. Available at: www.cdc.gov/drugresistance/. Accessed November 15, 2016.
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial resistance. Biggest threats. Available at: www.cdc.gov/drugresistance/biggest_threats.html. Accessed November 27, 2016.
- Fletcher-Lartey S, Yee M, Gaarsley C, et al. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study. BMJ Open. 2016;6:e012244.
- Urgent Care Association. Antibiotic prescriptions in urgent care. Available at: www.ucaoa.org/surveys/results.asp?qs=ed0f7e3383cb29. Accessed November 15, 2016.
- McKay R, Mah A, Law MR, et al. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrob Agents Chemother. 2016;60(7):4106-4018.
- Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR. Available at: www.cdc.gov/mmwr/volumes/65/rr/rr6506a1.htm. Accessed November 20, 2016.
- Fashner J, Ericson K, Werner S. Treatment of the common cold in children and adults. Am Fam Physician. 2012;86(2):153-159.
- Pratter MR. Cough and the common cold: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):72S-74S.
- Chow AW, Benninger MS, Brook I, e al. Infectious Diseases Society of America. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72–e112.
- Barlam TM, Morgan JR, Wetzler LM, et al. Antibiotics for respiratory tract infections: a comparison of prescribing in an outpatient setting. Infec Control Hosp Epidemiol. 2014;36(2):153-159.
- Infectious Diseases Society of America. Bad sore throat? It’s probably not strep, most likely viral. Available at: www.idsociety.org/2012_Strep_Throat_Guideline/. Accessed January 4, 2017.
- Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
- Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79(5):383-390.
- Fine AM, Nizet V, Mandl KD. Large-scale validation of the Centor and McIsaac scores to predict group A streptococcal pharyngitis. Arch Inter Med. 2012;172(11):847-852.
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat. 2014;10:1-171.
- Jorgenson LC, Christensin SF, Currea GC, et al. Antibiotic prescribing in patients with acute rhinosinusitis is not in agreement with European recommendations. Scand J Prim Health Care. 2013;31(2):101-105.
- Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2S):S1-S39.
- Wald ER, Applegate KE, Bordley C, et al. American Academy of Pediatrics. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132(1):e262–e280.
- Meltzer EO, Hamilos DL, Hadley JA. Rhinosinusitis: establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg. 2004;131(6)(Suppl):S1-S62.
- Evertsen J, Baumgardner DJ, Regnery A, Banerjee I. Diagnosis and management of pneumonia and bronchitis in outpatient primary care practices. Prim Care Respir J. 2010;19(3):237-241.
- Albert RH. Diagnosis and treatment of acute bronchitis. Am Fam Physician. 2010;82(11):1345-1350.
- Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough: ACCP evidence -based clinical practice guidelines. Chest. 2006;129(1 Suppl).
- Kincade S, Long NA. Acute bronchitis. Am Fam Physician. 2016 1;94(7):560-565.
- CD, Definitions, terminology, and classification. In: Rosenfeld RM, Bluestone CD, eds. Evidence-based otitis media. Hamilton, Canada: BC Decker; 2003:120–135.
- Lieberthal AS, Carroll AE, Chonmaitree T, et al. Clinical practice guideline: the diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–e999.
- Rettig E, Tunkel DE. Contemporary concepts in management of acute otitis media in children. Otolaryngol Clin North Am. 2014;47(5):651-672.
- Burrows HS, Blackwood RA, Cooke JM, et al. Otitis media guidelines for clinical care ambulatory. Faculty Practice Group University of Michigan Health System. April 2013.
- Urgent Care Association. Improving antibiotic stewardship in urgent care. Available at: http://c.ymcdn.com/sites/www.ucaoa.org/resource/resmgr/legislative/Antibiotic_Stewardship.pdf. Accessed January 14, 2017.
- The White House. Fact sheet: Obama administration release national action plan to combat antibiotic-resistant bacteria. Marcy 27, 2015. Available at: www.whitehouse.gov/the-press-office/2015/03/27/fact-sheet-obama-administration-releases-national-action-plan-combat-ant. Accessed November 15, 2016.
- Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance. Protecting patients and stopping outbreaks. Available at: www.cdc.gov/drugresistance/protecting_patients.html. Accessed January 13, 2017.
- Centers for Disease Control and Prevention Get Smart: Know when antibiotics work. Available at: www.cdc.gov/getsmart/community/programs-measurement/national-activities/antibiotics-work.html. Accessed January 13, 2017.
- Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance. Antibiotic resistance solutions initiative. Available at: www.cdc.gov/drugresistance/solutions-initiative/. Accessed January 13, 2017.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Executive summary: implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):1197-1202.
- Institute of Medicine. (2011). Clinical practice guidelines we can trust. National Academies Press, Washington, D.C. Available at: http://www.nap.edu/catalog/13058/clinical-practice-guidelines-we-can-trust
- Webber EC, Warhurst HM, Smith SS, et al. Conversion of a single-facility pediatric antimicrobial stewardship program to multi-facility application with computerized provider order entry and clinical decision support. Appl Clin Informatics. 2013;4:556-568.
- Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267-273.
- Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309:2345-2352.