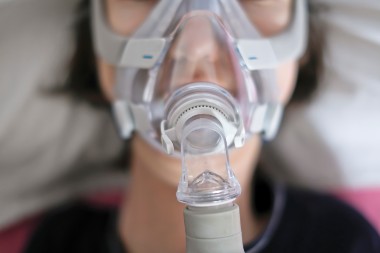
The Food and Drug Administration (FDA) issued a statement saying at least 561 deaths reported between April 2021 and September 2023 may be linked to Philips sleep apnea devices, typically referred to as “CPAP” (continuous positive airway pressure) machines. About 5 million of the devices were first recalled in 2021. The CPAP machines contain materials that have been found to deteriorate and cause serious health concerns. At issue is the polyester-based polyurethane (PE-PUR) foam components …
Read More