Urgent message: The approved use of monoclonal antibodies to treat patients who have COVID-19 may signal a shift from inpatient to outpatient care of infected individuals who do not require hospitalization. Urgent care facilities may be ideally suited to serve as treatment centers and to become destinations of choice for such patients.
Lindsey Fish, MD
Now that COVID-19 has been with us for over a year, we are in a much different position regarding the treatment of this illness. While many of the initial therapeutics were focused on inpatient, specifically ICU-level management, there has been continued research and interest in the outpatient management of COVID-19. Currently, the only approved outpatient treatment involves the use of monoclonal antibodies (mAb) which require intravenous infusion. This method of administration creates a logistical problem in most outpatient settings. However, as John Adams said, “Every problem is an opportunity in disguise.”
Urgent care centers now have the opportunity to be creative and take advantage of this rare situation. In many ways, urgent care is an ideal setting for the administration of mAb; offering this service has benefits for both patients and the UCC alike. Specifically, most urgent care operations have a high degree of comfort managing patients with COVID-19, and UC providers are highly experienced in delivering such care.
Additionally, many UC clinics already provide infusions in the form of IV fluids and antibiotics, and have many of the necessary resources and infrastructure. Our clinic was able to begin a program providing for the administration of these infusions in short order, supporting our patients and community by providing this resource.
As mAb therapy is not yet ubiquitous, let’s take a look at its administration in the context of COVID-19 in an urgent care center.
Currently, mAbs are the only antiviral therapy available for COVID-19–infected patients prior to hospitalization.1 On November 9, 2020, Eli Lilly received emergency use authorization (EUA) from the Food and Drug Administration for bamlanivimab. Bamlanivimab was approved as a one-time infusion for cases of non-hypoxic mild/moderate COVID-19 illness. The EUA was issued following a study on this treatment showed that the number needed-to-treat (NNT) to prevent a hospitalization/ED visit within 29 days was 21.3; that decreased to a number-needed-to treat of 9.6 specifically in high-risk patients (age>65, BMI>35).2
Additionally, patients receiving bamlanivimab felt better and had improvement of their symptoms as early as day 2 following administration.
Regeneron quickly followed on November 21, 2020, with an EUA for its mAb product, a combination of casirivimab and imdevimab. The FDA intervened immediately because the effect of the mAb products on mild-to-moderate COVID-19 illness showed clear benefits. As hospitals continued to be overwhelmed with COVID-19 patients, it became clear that preventing ED visits and hospitalization could not only meaningfully impact individual patient outcomes, but also reduce demands on the entire healthcare system. More recently, on February 9,2021 Eli Lilly received an EUA for its mAb combination product, bamlanivimab and etesevimab.
Administration of bamlanivimab, specifically its single preparation, is more straightforward. As such, it may be a better mAb option for UC clinics. Currently, the EUA for bamlanivimab approves its use for COVID-19 patients, within 10 days of symptom onset, not requiring oxygen therapy. In addition, the adult patient must have one of the following conditions: BMI ≥35, chronic kidney disease, diabetes, immunosuppressive disease, receiving immunosuppressive treatment, age ≥65 or age ≥55 with cardiovascular disease, hypertension, or COPD/chronic pulmonary disease.
The EUA also authorized the use of bamlanivimab in adolescents 12-17 years of age with one of the following conditions: BMI ≥85th percentile for age/gender, sickle cell disease, congenital or acquired heart disease, neurodevelopmental disorders (eg, cerebral palsy), medical-related technological dependence (eg, tracheostomy, gastrostomy, or positive pressure ventilation not related to COVID-19), or asthma, reactive airway, or other chronic respiratory disease requiring daily medication for control.3 The administration of bamlanivimab is via IV infusion. The treatment dose is 700 mg, provided in one vial. Initially, this had to be mixed into 250 mL of sodium chloride and infused over 60 minutes. However, now the 700 mg can be mixed into smaller amounts of sodium chloride and infused more rapidly. Please see Table 1.4
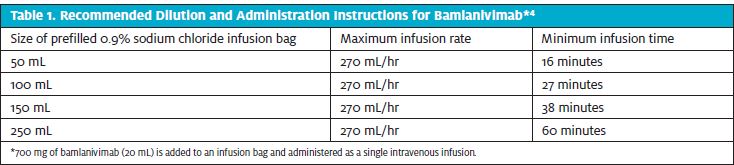
Monitoring of the patient is recommended during the infusion and for 60 minutes following the infusion. Adverse events occur in less than 3% of patients and most are mild and include hypersensitivity reactions, nausea/vomiting, diarrhea, dizziness, headache, and pruritis. If a mild infusion reaction occurs, it can usually be treated by slowing the infusion rate and providing symptom management. Providers should be aware that anaphylaxis is exceptionally rare but is a possibility. As such, the sites administering bamlanivimab should have a process and resources in place to manage this type of medical emergency.
Bamlanivimab needs to be stored in its original vial and protected from light at 2⁰-8⁰C. It should not be frozen, shaken, heated, or exposed to direct light. Once it is reconstituted, it is stable for 24 hours at 2⁰-8⁰C, or 7 hours at room temperature (including infusion time). It should be warmed to room temperature for at least 15 minutes prior to administration. Infusion supplies required to administer bamlanivimab include an IV insertion kit, sodium chloride bag, IV infusion tubing, and a filter. If an UC clinic does not have a pump available, bamlanivimab can also be hung to gravity and the drip rate can be used to calculate the infusion rate.
Patients receiving the infusion should be provided the bamlanivimab fact sheet.5 Providers should have a conversation with the patient regarding monoclonal antibodies and how they work. A formal informed consent process discussing the risks and alternatives is recommended. In our clinic, following the administration of 90 doses of bamlanivimab to qualified patients, only two patients presented to the ED/hospital with worsening symptoms and only one patient experienced a mild adverse reaction.
Distribution of the mAbs has been managed by Operation Warp Speed (OWS). The United States Federal Government has distributed the supply to the state and territory health departments and has left final allocation responsibility to them. The distribution supply has been determined by confirmed hospitalizations and confirmed cases in an effort to ensure both temporal and geographic equity.6 This has led to some difficulty in smaller, non–hospital-based systems receiving doses.
Additionally, OWS has created the Special Projects for Equitable and Efficient Distribution (SPEED) program, which launched in mid-December. This program has provided direct distribution to long-term care facilities, federally qualified health centers, dialysis centers, and correctional facilities.7 As such, urgent care centers interested in offering mAb infusions should contact their state/territory health department to determine how to apply for a supply. UCCs might also partner with programs able to receive a direct distribution via SPEED in order to help administer mAbs to their patients.
The United States Federal Government has purchased all of the mAbs directly from the manufacturers. As such, the medication itself is free to patients and providers. The Centers for Medicare and Medicaid Services has set a fully loaded reimbursement rate on average at $309.60 to cover the administration cost in all settings.8 In addition, these infusions are required to be covered by state Medicaid as part of the Families First Coronavirus Response Act.9 It is anticipated that commercial insurers will follow the lead of CMS in regard to reimbursement. Details regarding coding and billing are available through CMS.gov.8
We will continue to see COVID-19 infections in our urgent care centers, and as we are accustomed to seeing these patients for their illness, we now have the opportunity to begin offering treatment for certain higher-risk individuals as well. Early data suggest that treating these infections with mAb infusions early in the course may result in better patient outcomes and reduce need for ICU-level care in our health system.
In our clinic, when patients who would qualify for mAb therapy present with possible COVID-19, we perform a rapid COVID-19 PCR test. If the test is positive, we immediately administer bamlanivimab in accordance with our state requirements. The patient and provider alike are generally thrilled to have a successful and complete visit. There are many resources available to guide clinics in establishing their own procedures to perform these infusions when indicated.4,10,11 As such, mAb therapy represents another clear opportunity for urgent care to serve patients and public health alike in the setting of this crisis.
References
- Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19. Accessed April 9, 2021.
- Chen P, Nirula A, Heller B, et al for the BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237.
- Eli Lilly and Company. Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Bamlanivimab. Available at: https://pi.lilly.com/eua/bamlanivimab-eua-factsheet-hcp.pdf. Accessed April 9. 2021.
- Food and Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab. Available at: https://www.fda.gov/media/143603/download. Accessed April 9, 2021.
- Eli Lilly and Company. Fact Sheet for Patients, Parents and Caregivers Emergency Use Authorization (EUA) of Bamlanivimab for Coronavirus Disease 2019 (COVID-19) Available at: bamlanivimab-eua-factsheet-patient.pdf (lilly.com). Accessed April 9, 2021.
- U.S. Department of Health and Human Services. Outpatient monoclonal antibody treatment for COVID-19 made available under Emergency Use Authorization. Available at: www.phe.gov/bamlanivimab. Accessed April 9, 2021.
- U.S. Department of Health and Human Services. Special Projects for Equitable and Efficient Distribution (SPEED) of COVID-19 Outpatient Therapeutics. Available at: https://www.phe.gov/emergency/events/COVID19/investigation-MCM/Pages/SPEED.aspx. Accessed April 9, 2021.
- Centers for Medicare and Medicaid Services. Medicare Monoclonal Antibody COVID-19 Infusion Program Instruction. Available at: https://www.cms.gov/files/document/covid-medicare-monoclonal-antibody-infusion-program-instruction.pdf. Accessed April 9, 2021.
- Centers for Medicare and Medicaid Services. Coverage of Monoclonal Antibody Products to Treat COVID-19. Available at: https://www.cms.gov/files/document/covid-infographic-coverage-monoclonal-antibody-products-treat-covid-19.pdf. Accessed April 9, 2021.
- U.S. Department of Health and Human Services. Therapeutics: Monoclonal Antibody Playbook. https://www.phe.gov/emergency/events/COVID19/investigation-MCM/Documents/COVID-Therapeutics-playbook_1Feb2021.pdf. Accessed April 9, 2021.
- Eli Lilly and Company. Lilly Bamlanivimab Antibody Playbook. Available at: https://www.covid19.lilly.com/assets/pdf/bamlanivimab/lilly-antibodies-playbook.pdf. Accessed April 9, 2021.

