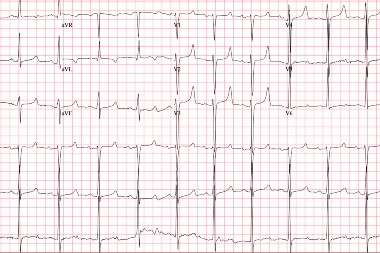
A 35-year-old male with a history of end-stage-renal-disease (ESRD) presents to urgent care complaining of back pain. The patient missed his dialysis session today because of the pain. An ECG is obtained. View the ECG captured below and consider what your diagnosis and next steps would be. Resolution of the case is described on the next page.
Read More