Published on
Urgent message: Respiratory complaints due to use of e-cigarettes or vaping may be difficult to distinguish from those associated with influenza and other common respiratory illnesses. It is essential for the urgent care provider to ask patients who present with any respiratory complaint about vaping history, and to have a sound understanding of next steps for patients suspected of having e-cigarette or vaping-associated lung injury (EVALI).
Marco Propersi, DO and Anand Swaminathan, MD, MPH
Citation: Propersi M, Swaminathan A. Vaping-associated lung injury (EVALI) presenting to urgent care. J Urgent Care Med. 2021;15(4):13-16.
INTRODUCTION
E-cigarette or vaping-associated lung injury (EVALI) disproportionately affects teenagers and younger adults due to increased incidence of vaping in these age groups.1 While this article will focus on the link between EVALI and vaping with tetrahydrocannabinol (THC) containing products,2 it is important to acknowledge that vaping with nicotine or other products is also linked to EVALI.
ILLUSTRATIVE CASE
A 22-year-old man presents to an urgent care with 2 days of subjective fevers and chills, dry cough, and shortness of breath. He used over-the-counter cough and cold medications for fever and cough suppression with minimal relief. His vital signs are notable for temp of 100.1⁰, HR of 105 beats/min, RR of 20 breaths/min, blood pressure of 118/75 mmHg, pulse oximeter of 96% on room air. He is nontoxic appearing and has an occasional cough. His lungs are clear, there are no rales, rhonchi, or wheezes. There is no accessory muscle use or respiratory distress. He speaks in full sentences without dyspnea. There is mild tachypnea. A social history is not obtained.
Urgent Care Management
The patient was diagnosed with bronchitis and given azithromycin 5-day dose pack and an albuterol inhaler. Radiographic imaging was forgone at this time. Return precautions were given and the patient was advised to return in 7 days for reevaluation.
Outcome
Over the next 24 hours the patient’s condition worsened prompting him to present to the emergency department. On reassessment in the ED, the patient now had markedly unstable vital signs. His temp was 103.5⁰, HR of 130 beats/min, RR of 30 breaths/min, blood pressure of 148/92 mmHg, and pulse oximeter of 88% on room air. On physical exam he was in obvious respiratory distress with moderate tachypnea and scattered rales and rhonchi throughout all lung fields. His pulse oximeter improved to 99% after being placed on a 15 L/min nonrebreather. A chest x-ray was performed but was unremarkable. A CT of the chest was then obtained, revealing revealed scattered ground glass opacities suspicious for inhalation injury. (See Figure 1 and Figure 2.)
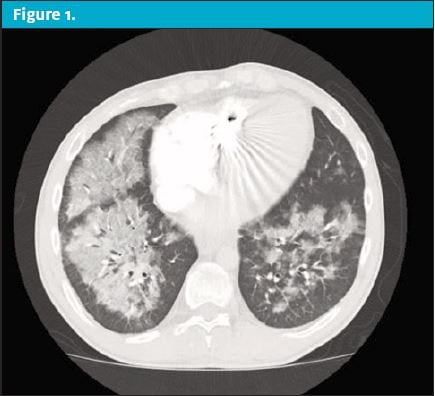
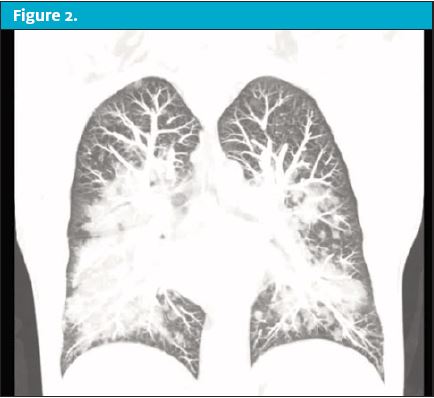
Upon questioning, the patient admitted to vaping with THC products. The patient was presumed to have EVALI. He was started on systemic glucocorticoids and antibiotics with coverage against community acquired pneumonia. He was admitted to the intensive care unit for supportive respiratory care.
Over the next week the patient’s condition improved and his oxygen requirements returned to normal. Testing for other infectious processes was normal. He was counseled on smoking cessation behaviors and vaping with recreational substances and additives and was discharged with outpatient follow-up.
DISCUSSION
E-cigarettes are battery operated electronic devices which aerosolize a liquid solution containing nicotine for inhalation. The first U.S. patent appeared in 1963 as a “a form of smokeless tobacco” with the aim of providing ”a safe and harmless means for smoking.” The term “vaping” is a misnomer because the e-cigarette solution is actually aerosolized and not vaporized. There are multiple generations of devices but all have the same basic construction. The devices consist of a mouthpiece, a chamber which houses the e-cigarette solution, an atomizer which aerosolizes the solution, and a battery.3
The e-cigarette solution can contain numerous potentially harmful compounds including nicotine, propylene glycol, heavy metals, formaldehyde, and one of more than 7,000 flavors.3,4 Additionally, unintended compounds such as bacterial endotoxin and components of fungal cell walls have also been found in the solution.5 E-cigarette devices are often modified to aerosolize THC containing solutions whose components may not be known.
Epidemiology
EVALI was first recognized in the summer of 2019. It peaked in August of 2019 and has steadily declined since. Prior to the decline, information was gleaned from a few published case reports.12,13,14,15 As per the final CDC report on February 18, 2020, there have been more than 2,800 cases of EVALI and 68 deaths in the U.S.6 (See Figure 3.)
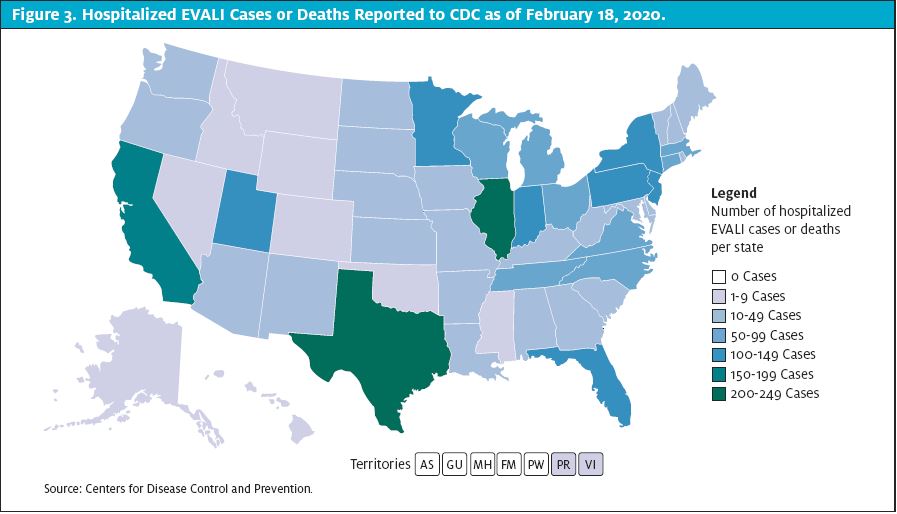
The signs and symptoms can mimic many common respiratory conditions making diagnosis difficult. As clinicians, there’s less impetus to report and publish mild cases where the patient’s condition improved without dramatic rescue efforts. There have likely been many more patients who were either misdiagnosed or did not seek medical treatment and symptoms improved without medical intervention. Among the cases reported to the CDC, 66% were male, and 74% were 34-years-old or younger. The age range was 13-85 and the median age was 24-years-old. Only 2% of cases, but nearly 25% of deaths, occurred in patients older than 65-years-of-age. The median age of deceased patients was 49.5-years-old.6
Among cases that had data on substance use, 82% reported use of THC-containing products. However, 14% reported exclusive use of nicotine products6 (though it is possible some patients may have been apprehensive about reporting illegal substance use). Additionally, in some cases information on substance use was obtained from family or proxies who may not have been aware of the patient’s full scope of substance use.
There is compelling evidence that vitamin E is the causative agent. Vitamin E acetate has been used as a dissolvent in THC-containing solutions.7 Vitamin E acetate is found in many commercially available products, most notably foods and cosmetic products. It is safe when ingested or applied to the skin. However, recent evidence has linked inhalation of vitamin E acetate to EVALI. In one study, vitamin E acetate was identified in bronchoalveolar lavage specimens from 29 patients with EVALI in 10 different states.8 In another study of 51 patients with EVALI across 16 states, vitamin E acetate was detected in 94% of BAL specimens.9 The exact mechanism by which vitamin E causes pulmonary toxicity is unknown.
Clinical Symptoms and Physical Exam
The signs and symptoms of EVALI are nonspecific and vary considerably from one patient to the next. It may be impossible to clinically distinguish EVALI from other common respiratory conditions such as influenza, pneumonia or the common cold. Much of what we know comes from CDC reports and a few small case series, the largest of which contained just under 100 patients.2,10,11 All patients (100%) experienced some constitutional symptoms with subjective fever (84%) and chills (60%) being the most common.2 Nearly all patients (97%) had respiratory symptoms. Shortness of breath and cough were each present in 85% of patients.2 Most patients (77%) experienced gastrointestinal symptoms; nausea (66%) and vomiting (61%) were the most common.2
On physical exam, fever (33%), tachycardia (63%), and tachypnea (43%) were seen to varying degrees.2 More than half the patients were hypoxemic (58%) and one fourth had oxygen saturations <88% at presentation.2 There is no single exam finding that is pathognomonic for EVALI.
Diagnostics
Labs
There is no single lab test that is indicative of EVALI; it remains a diagnosis of exclusion. Testing is directed towards ruling out other potential causes of disease such as cardiac, rheumatologic, neoplastic and infectious causes.10
Imaging
The CDC-proposed confirmed case definition requires use of e-cigarettes in preceding 90 days, pulmonary infiltrates on chest imaging, and absence of alternative plausible diagnosis. One hundred percent of patients with EVALI will have bilateral ground glass opacities on CT imaging,2,10,11 but the chest x-ray is less sensitive, with only 83% demonstrating infiltrates.2 In stable patients, with a clear story suggestive of EVALI and infiltrates on chest x-ray, a chest CT would not significantly alter management and is not required for diagnosis. EVALI has been linked to lipoid pneumonia but a variety of other radiographic patterns can be seen including diffuse alveolar hemorrhage, acute eosinophilic pneumonia, hypersensitivity pneumonitis, and organizing pneumonia.12-15
Treatment
There is no approved treatment for EVALI. Patients should receive antibiotics against bacterial pneumonia until infectious processes have been ruled out. Most patients with EVALI have received corticosteroids.2,10,11 In one study, ICU patients received 125 mg IV methylprednisolone daily for 15 days; patients admitted to the medical ward received 125 mg IV methylprednisolone daily for 10 days; and those managed outpatient received 50 mg PO prednisone daily for 7 days.11 However, the benefits of systemic steroid use are based on observation and have not been formally studied. In previous studies, most patients (95%) with EVALI required hospitalization for management of hypoxemia.2 Supplemental oxygen should be given to maintain oxygen saturation >92%. Escalation of respiratory support may be required. Many patients may need mechanical ventilation and there are case reports of patients requiring ECMO.13
TEACHING POINTS
- EVALI may be indistinguishable from influenza and other common respiratory complaints.
- Ask patients with respiratory complaints about vaping history, especially with THC products.
- Chest x-ray will often show bilateral opacities.
- Start patients on antibiotics until infection is ruled out.
- Hospitalized patients should receive broad-spectrum antibiotics.
- Those being managed outpatient should be treated for community-acquired pneumonia. Amoxicillin plus azithromycin or doxycycline, amoxicillin clavulanate, or cefpodoxime plus azithromycin or doxycycline are all appropriate regimens.
- Corticosteroids may be helpful.
- Many patients will require hospitalization and respiratory support.
REFERENCES
- Wang TW, Gentzke AS, Creamer MR, et al. Tobacco product use and associated factors among middle and high school students – United States, 2019. MMWR Surveill Summ. 2019;68(12):1–22.
- Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin – final report. N Engl J Med. 2020;382(10):903–916.
- U.S. Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the surgeon general. Available at: https://www.cdc.gov/tobacco/data_statistics/sgr/e-cigarettes/pdfs/2016_sgr_entire_report_508.pdf. Accessed November 20, 2020.
- Olmedo P, Goessler W, Tanda S, et al. Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environ Health Perspect. 2018;126(2):027010.
- Lee MS, Allen JG, Christiani DC. Endotoxin and (1[R]3)-b-D-glucan contamination in electronic cigarette products sold in the United States. Environ Health Perspect. 2019;127(4):47008.
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Available at: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed December 10, 2020.
- Pray IW, Atti SK, Tomasallo C, Meiman JG. E-cigarette, or vaping, product use-associated lung injury among clusters of patients reporting shared product use – Wisconsin, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(9):236–240.
- Blount BC, Karwowski MP, Morel-Espinosa M, et al. Evaluation of bronchoalveolar lavage fluid from patients in an outbreak of e-cigarette, or vaping, product use-associated lung injury – 10 States, August-October 2019. MMWR Morb Mortal Wkly Rep. 2019;68(45):1040–1041. [Erratum: MMWR Morb Mortal Wkly Rep. 2020;69(4):116.]
- Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697–705.
- Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017–1026.
- Blagev DP, Harris D, Dunn AC, et al. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet. 2019;394(10214):2073–2083.
- Agustin M, Yamamoto M, Cabrera F, Eusebio R. Diffuse Alveolar Hemorrhage Induced by Vaping. Case Rep Pulmonol. 2018;2018:9724530.
- Aokage T, Tsukahara K, Fukuda Y, et al. Heat-not-burn cigarettes induce fulminant acute eosinophilic pneumonia requiring extracorporeal membrane oxygenation. Respir Med Case Rep. 2018;26:87–90.
- Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia – North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784–786.
- Khan MS, Khateeb F, Akhtar J, et al. Organizing pneumonia related to electronic cigarette use: A case report and review of literature. Clin Respir J. 2018;12(3):1295–1299.
Author affiliations: Marco Propersi, DO, FAAEM, St. Joseph’s Regional Medical Center. Anand Swaminathan, MD, MPH, St. Joseph’s Regional Medical Center. The authors have no relevant financial relationships with any commercial interests.
